NEET-XII-Chemistry
02: Solutions
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #9Vapour
pressure of pure water at 298 K is 23.8 mm Hg. 50 g of urea
(NH2CONH2)
is dissolved in 850 g of water. Calculate the vapour pressure of
water for this solution and its relative lowering.Ans : It
is given that vapour pressure of water,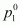 =
=
23.8 mm of Hg
Weight
of water taken, w1 = 850 g
Weight
of urea taken, w2 = 50 g
Molecular
weight of water, M1 = 18 g mol-1
Molecular
weight of urea, M2 = 60 g mol-1
Now,
we have to calculate vapour pressure of water in the solution. We
take vapour pressure as p1.
Now,
from Raoult’s law, we have:
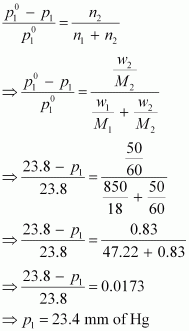
Hence,
the vapour pressure of water in the given solution is 23.4 mm of Hg
and its relative lowering is 0.0173.
- Qstn #10Boiling
point of water at 750 mm Hg is 99.63°C. How much sucrose is to be
added to 500 g of water such that it boils at 100°C.
Molal elevation constant for water is 0.52 K kg mol-1.Ans : Here,
elevation of boiling point ΔTb = (100 + 273) - (99.63 + 273)
=
0.37 K
Mass
of water, wl = 500 g
Molar
mass of sucrose (C12H22O11), M2 = 11 × 12 + 22 × 1 + 11 × 16
=
342 g mol-1
Molal
elevation constant, Kb = 0.52 K kg mol-1
We
know that:
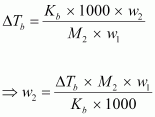
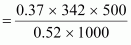
=
121.67 g (approximately)
Hence,
121.67 g of sucrose is to be added.
Note: There is a slight variation in this answer and the one given in the
NCERT textbook.
- Qstn #11Calculate
the mass of ascorbic acid (Vitamin C, C6H8O6)
to be dissolved in
75 g of acetic acid to lower its melting point
by 1.5°C. Kf = 3.9 K kg mol-1.Ans : Mass
of acetic acid, w1 = 75 g
Molar
mass of ascorbic acid (C6H8O6), M2 = 6 × 12 + 8 × 1 + 6 × 16
=
176 g mol-1
Lowering
of melting point, ΔTf = 1.5 K
We
know that:
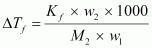
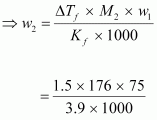
= 5.08 g
(approx)
Hence,
5.08 g of ascorbic acid is needed to be dissolved.
Note: There is a slight variation in this answer and the one given in the
NCERT textbook.
- Qstn #12Calculate
the osmotic pressure in pascals exerted by a solution prepared by
dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water
at 37°C.Ans : It is
given that:
Volume
of water, V = 450 mL = 0.45 L
Temperature, T = (37 +
273)K = 310 K
Number
of moles of the polymer,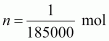
We know
that:
Osmotic
pressure,
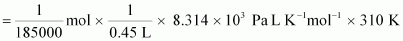
= 30.98 Pa
= 31
Pa (approximately)
- #Section : BPage No 59:
- Qstn #1Define
the term solution. How many types of solutions are formed? Write
briefly about each type with an example.Ans : Homogeneous mixtures of
two or more than two components are known as solutions.
There are three types
of solutions.
(i) Gaseous
solution:
The
solution in which the solvent is a gas is called a gaseous solution.
In these solutions, the solute may be liquid, solid, or gas. For
example, a mixture of oxygen and nitrogen gas is a gaseous solution.
(ii) Liquid
solution:
The
solution in which the solvent is a liquid is known as a liquid
solution. The solute in these solutions may be gas, liquid, or solid.
For
example, a solution of ethanol in water is a liquid solution.
(iii) Solid
solution:
The
solution in which the solvent is a solid is known as a solid
solution. The solute may be gas, liquid or solid. For example, a
solution of copper in gold is a solid solution.
- Qstn #2Give
an example of solid solution in which the solute is a gas.Ans : In case a
solid solution is formed between two substances (one having very
large particles and the other having very small particles), an
interstitial solid solution will be formed. For example, a solution
of hydrogen in palladium is a solid solution in which the solute is a
gas.
- Qstn #3Define
the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
(iv) Mass percentage.Ans : (i) Mole fraction:
The
mole fraction of a component in a mixture is defined as the ratio of
the number of moles of the component to the total number of moles of
all the components in the mixture.
i.e.,
Mole
fraction of a component
Mole
fraction is denoted by ‘x’.
If
in a binary solution, the number of moles of the solute and the
solvent are nA and nB respectively, then the mole fraction of the solute in the solution is
given by,
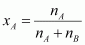
Similarly,
the mole fraction of the solvent in the solution is given as:

(ii) Molality
Molality
(m) is defined as the number of moles of the solute per kilogram of
the solvent. It is expressed as:
Molality
(m)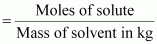
(iii) Molarity
Molarity
(M) is defined as the number of moles of the solute dissolved in one
Litre of the solution.
It
is expressed as:
Molarity
(M)
(iv) Mass
percentage:
The
mass percentage of a component of a solution is defined as the mass
of the solute in grams present in 100 g of the solution. It is
expressed as:
Mass
% of a component
- Qstn #4Concentrated
nitric acid used in laboratory work is 68% nitric acid by mass in
aqueous solution. What should be the molarity of such a sample of the
acid if the density of the solution is 1.504 g mL-1?Ans : Concentrated nitric
acid used in laboratory work is 68% nitric acid by mass in an aqueous
solution. This means that 68 g of nitric acid is dissolved in 100 g
of the solution.
Molar mass of nitric
acid (HNO3) = 1 ×
1 + 1 × 14 + 3 ×
16 = 63 g mol-1
Then, number of moles
of HNO3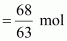
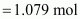
Given,
Density of solution =
1.504 g mL-1
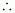 Volume
Volume
of 100 g solution =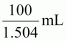

Molarity of solution

Page No 60:
- Qstn #5A
solution of glucose in water is labelled as 10% w/w, what would be
the molality and mole fraction of each component in the solution? If
the density of solution is 1.2 g mL-1,
then what shall be the molarity of the solution?Ans : 10% w/w solution of
glucose in water means that 10 g of glucose in present in 100 g of
the solution i.e., 10 g of glucose is present in (100 - 10) g =
90 g of water.
Molar mass of glucose
(C6H12O6) = 6 ×
12 + 12 × 1 + 6 ×
16 = 180 g mol-1
Then, number of moles
of glucose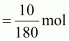
=
0.056 mol
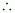 Molality
Molality
of solution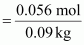 =
=
0.62 m
Number of moles of
water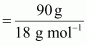
= 5 mol
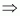 Mole
Mole
fraction of glucose
And, mole fraction of
water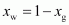
= 1 - 0.011
= 0.989
If the density of the
solution is 1.2 g mL-1, then the volume of the 100 g
solution can be given as:
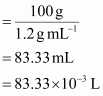
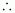 Molarity
Molarity
of the solution
= 0.67 M
- Qstn #6How
many mL of 0.1 M HCl are required to react completely with 1 g
mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?Ans : Let the amount of
Na2CO3 in the mixture be x g.
Then, the amount of
NaHCO3 in the mixture is (1 - x) g.
Molar mass of Na2CO3 = 2 × 23 + 1 × 12 + 3 × 16
= 106 g mol-1
 Number of moles Na2CO3
Number of moles Na2CO3 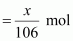
Molar mass of NaHCO3 = 1 × 23 + 1 × 1 × 12 + 3 × 16
= 84 g mol-1
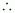 Number
Number
of moles of NaHCO3
According to the
question,
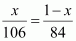
⇒ 84x =
106 - 106x
⇒ 190x =
106
⇒ x =
0.5579
Therefore, number of
moles of Na2CO3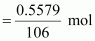
= 0.0053 mol
And, number of moles of
NaHCO3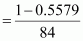
= 0.0053 mol
HCl reacts with Na2CO3 and NaHCO3 according to the following equation.
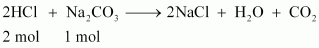

1 mol of Na2CO3 reacts with 2 mol of HCl.
Therefore, 0.0053 mol
of Na2CO3 reacts with 2 × 0.0053 mol =
0.0106 mol.
Similarly, 1 mol of
NaHCO3 reacts with 1 mol of HCl.
Therefore, 0.0053 mol
of NaHCO3 reacts with 0.0053 mol of HCl.
Total moles of HCl
required = (0.0106 + 0.0053) mol
= 0.0159 mol
In 0.1 M of HCl,
0.1 mol of HCl is
preset in 1000 mL of the solution.
Therefore, 0.0159 mol
of HCl is present in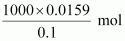
= 159 mL of the
solution
Hence, 159 mL of 0.1 M
of HCl is required to react completely with 1 g
mixture of Na2CO3 and NaHCO3, containing equimolar amounts of both.
- Qstn #7A
solution is obtained by mixing 300 g of 25% solution and 400 g of 40%
solution by mass. Calculate the mass percentage of the resulting
solution.Ans : Total amount of solute
present in the mixture is given by,
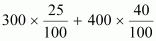
= 75 + 160
= 235 g
Total amount of
solution = 300 + 400 = 700 g
Therefore, mass
percentage (w/w) of the solute in the resulting solution,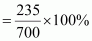
= 33.57%
And, mass percentage
(w/w) of the solvent in the resulting solution,
= (100 - 33.57)%
= 66.43%
- Qstn #8An
antifreeze solution is prepared from 222.6 g of ethylene glycol
(C2H6O2)
and 200 g of water. Calculate the molality of the solution. If the
density of the solution is 1.072 g mL-1,
then what shall be the molarity of the solution?Ans : Molar mass of ethylene
glycol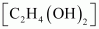 =
=
2 × 12 + 6 × 1 + 2 ×16
= 62 gmol-1
Number of moles of
ethylene glycol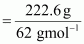
= 3.59 mol
Therefore, molality of
the solution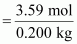
= 17.95 m
Total mass of the
solution = (222.6 + 200) g
= 422.6 g
Given,
Density of the solution
= 1.072 g mL-1
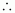 Volume
Volume
of the solution
= 394.22 mL
= 0.3942 × 10-3 L
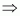 Molarity of the solution
Molarity of the solution 
= 9.11 M
- Qstn #9A sample of drinking water was found to be severely contaminated with chloroform (CHCl3) supposed to be a carcinogen. The level of contamination was 15 ppm (by mass):
(i) express this in percent by mass
(ii) determine the molality of chloroform in the water sample.digAnsr: bAns : (i) 15 ppm (by mass) means 15 parts per million (106) of the solution.
Therefore, percent by mass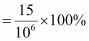
= 1.5 × 10-3 %
(ii) Molar mass of chloroform (CHCl3) = 1 × 12 + 1 × 1 + 3 × 35.5
= 119.5 g mol-1
Now, according to the question,
15 g of chloroform is present in 106 g of the solution.
i.e., 15 g of chloroform is present in (106 - 15) ≈ 106 g of water.
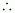 Molality of the solution
Molality of the solution
= 1.26 × 10-4 m
- Qstn #10What
role does the molecular interaction play in a solution of alcohol and
water?Ans : In pure alcohol and
water, the molecules are held tightly by a strong hydrogen bonding.
The interaction between the molecules of alcohol and water is weaker
than alcohol-alcohol and water-water interactions. As a
result, when alcohol and water are mixed, the intermolecular
interactions become weaker and the molecules can easily escape. This
increases the vapour pressure of the solution, which in turn lowers
the boiling point of the resulting solution.