NEET-XII-Chemistry
02: Solutions
- #4Concentrated
nitric acid used in laboratory work is 68% nitric acid by mass in
aqueous solution. What should be the molarity of such a sample of the
acid if the density of the solution is 1.504 g mL-1?Ans : Concentrated nitric
acid used in laboratory work is 68% nitric acid by mass in an aqueous
solution. This means that 68 g of nitric acid is dissolved in 100 g
of the solution.
Molar mass of nitric
acid (HNO3) = 1 ×
1 + 1 × 14 + 3 ×
16 = 63 g mol-1
Then, number of moles
of HNO3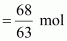
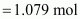
Given,
Density of solution =
1.504 g mL-1
 Volume
Volume
of 100 g solution =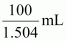

Molarity of solution

Page No 60: