NEET-XII-Chemistry
02: Solutions
- #5A
solution of glucose in water is labelled as 10% w/w, what would be
the molality and mole fraction of each component in the solution? If
the density of solution is 1.2 g mL-1,
then what shall be the molarity of the solution?Ans : 10% w/w solution of
glucose in water means that 10 g of glucose in present in 100 g of
the solution i.e., 10 g of glucose is present in (100 - 10) g =
90 g of water.
Molar mass of glucose
(C6H12O6) = 6 ×
12 + 12 × 1 + 6 ×
16 = 180 g mol-1
Then, number of moles
of glucose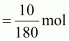
=
0.056 mol
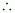 Molality
Molality
of solution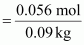 =
=
0.62 m
Number of moles of
water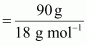
= 5 mol
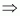 Mole
Mole
fraction of glucose
And, mole fraction of
water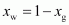
= 1 - 0.011
= 0.989
If the density of the
solution is 1.2 g mL-1, then the volume of the 100 g
solution can be given as:
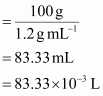
 Molarity
Molarity
of the solution
= 0.67 M