NEET-XII-Chemistry
02: Solutions
- #6How
many mL of 0.1 M HCl are required to react completely with 1 g
mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?Ans : Let the amount of
Na2CO3 in the mixture be x g.
Then, the amount of
NaHCO3 in the mixture is (1 - x) g.
Molar mass of Na2CO3 = 2 × 23 + 1 × 12 + 3 × 16
= 106 g mol-1
 Number of moles Na2CO3
Number of moles Na2CO3 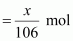
Molar mass of NaHCO3 = 1 × 23 + 1 × 1 × 12 + 3 × 16
= 84 g mol-1
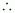 Number
Number
of moles of NaHCO3
According to the
question,
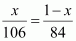
⇒ 84x =
106 - 106x
⇒ 190x =
106
⇒ x =
0.5579
Therefore, number of
moles of Na2CO3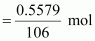
= 0.0053 mol
And, number of moles of
NaHCO3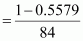
= 0.0053 mol
HCl reacts with Na2CO3 and NaHCO3 according to the following equation.
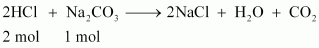

1 mol of Na2CO3 reacts with 2 mol of HCl.
Therefore, 0.0053 mol
of Na2CO3 reacts with 2 × 0.0053 mol =
0.0106 mol.
Similarly, 1 mol of
NaHCO3 reacts with 1 mol of HCl.
Therefore, 0.0053 mol
of NaHCO3 reacts with 0.0053 mol of HCl.
Total moles of HCl
required = (0.0106 + 0.0053) mol
= 0.0159 mol
In 0.1 M of HCl,
0.1 mol of HCl is
preset in 1000 mL of the solution.
Therefore, 0.0159 mol
of HCl is present in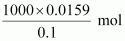
= 159 mL of the
solution
Hence, 159 mL of 0.1 M
of HCl is required to react completely with 1 g
mixture of Na2CO3 and NaHCO3, containing equimolar amounts of both.