NEET-XII-Chemistry
02: Solutions
- #9Vapour
pressure of pure water at 298 K is 23.8 mm Hg. 50 g of urea
(NH2CONH2)
is dissolved in 850 g of water. Calculate the vapour pressure of
water for this solution and its relative lowering.Ans : It
is given that vapour pressure of water,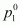 =
=
23.8 mm of Hg
Weight
of water taken, w1 = 850 g
Weight
of urea taken, w2 = 50 g
Molecular
weight of water, M1 = 18 g mol-1
Molecular
weight of urea, M2 = 60 g mol-1
Now,
we have to calculate vapour pressure of water in the solution. We
take vapour pressure as p1.
Now,
from Raoult’s law, we have:
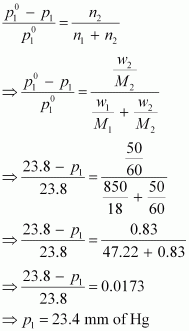
Hence,
the vapour pressure of water in the given solution is 23.4 mm of Hg
and its relative lowering is 0.0173.