NEET-XII-Chemistry
02: Solutions
- #8An
antifreeze solution is prepared from 222.6 g of ethylene glycol
(C2H6O2)
and 200 g of water. Calculate the molality of the solution. If the
density of the solution is 1.072 g mL-1,
then what shall be the molarity of the solution?Ans : Molar mass of ethylene
glycol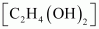 =
=
2 × 12 + 6 × 1 + 2 ×16
= 62 gmol-1
Number of moles of
ethylene glycol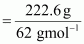
= 3.59 mol
Therefore, molality of
the solution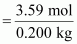
= 17.95 m
Total mass of the
solution = (222.6 + 200) g
= 422.6 g
Given,
Density of the solution
= 1.072 g mL-1
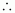 Volume
Volume
of the solution
= 394.22 mL
= 0.3942 × 10-3 L
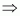 Molarity of the solution
Molarity of the solution 
= 9.11 M