NEET-XII-Chemistry
02: Solutions
- #12Calculate
the osmotic pressure in pascals exerted by a solution prepared by
dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water
at 37°C.Ans : It is
given that:
Volume
of water, V = 450 mL = 0.45 L
Temperature, T = (37 +
273)K = 310 K
Number
of moles of the polymer,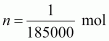
We know
that:
Osmotic
pressure,
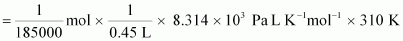
= 30.98 Pa
= 31
Pa (approximately)