NEET-XII-Chemistry
02: Solutions
- #7A
solution is obtained by mixing 300 g of 25% solution and 400 g of 40%
solution by mass. Calculate the mass percentage of the resulting
solution.Ans : Total amount of solute
present in the mixture is given by,
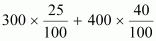
= 75 + 160
= 235 g
Total amount of
solution = 300 + 400 = 700 g
Therefore, mass
percentage (w/w) of the solute in the resulting solution,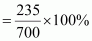
= 33.57%
And, mass percentage
(w/w) of the solvent in the resulting solution,
= (100 - 33.57)%
= 66.43%