NEET-XII-Chemistry
02: Solutions
- #9A sample of drinking water was found to be severely contaminated with chloroform (CHCl3) supposed to be a carcinogen. The level of contamination was 15 ppm (by mass):
(i) express this in percent by mass
(ii) determine the molality of chloroform in the water sample.digAnsr: bAns : (i) 15 ppm (by mass) means 15 parts per million (106) of the solution.
Therefore, percent by mass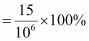
= 1.5 × 10-3 %
(ii) Molar mass of chloroform (CHCl3) = 1 × 12 + 1 × 1 + 3 × 35.5
= 119.5 g mol-1
Now, according to the question,
15 g of chloroform is present in 106 g of the solution.
i.e., 15 g of chloroform is present in (106 - 15) ≈ 106 g of water.
 Molality of the solution
Molality of the solution
= 1.26 × 10-4 m