NEET-XII-Chemistry
02: Solutions
- #3Define
the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
(iv) Mass percentage.Ans : (i) Mole fraction:
The
mole fraction of a component in a mixture is defined as the ratio of
the number of moles of the component to the total number of moles of
all the components in the mixture.
i.e.,
Mole
fraction of a component
Mole
fraction is denoted by ‘x’.
If
in a binary solution, the number of moles of the solute and the
solvent are nA and nB respectively, then the mole fraction of the solute in the solution is
given by,
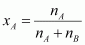
Similarly,
the mole fraction of the solvent in the solution is given as:

(ii) Molality
Molality
(m) is defined as the number of moles of the solute per kilogram of
the solvent. It is expressed as:
Molality
(m)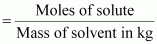
(iii) Molarity
Molarity
(M) is defined as the number of moles of the solute dissolved in one
Litre of the solution.
It
is expressed as:
Molarity
(M)
(iv) Mass
percentage:
The
mass percentage of a component of a solution is defined as the mass
of the solute in grams present in 100 g of the solution. It is
expressed as:
Mass
% of a component