NEET-XI-Chemistry
05: States of Matter
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- #Chapter 5 - States of Matter
- Qstn #1What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
Ans : Given,
Initial pressure, p1 = 1 bar
Initial volume, V1 = 500 dm3
Final volume, V2 = 200 dm3
Since the temperature remains constant, the final pressure (p2) can be calculated using Boyle’s law.
According to Boyle’s law,
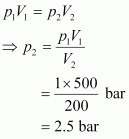
Therefore, the minimum pressure required is 2.5 bar.
- Qstn #2A vessel of 120 mL capacity contains a certain amount of gas at 35 °C and 1.2 bar pressure. The gas is transferred to another vessel of volume 180 mL at 35 °C. What would be its pressure?
Ans : Given,
Initial pressure, p1 = 1.2 bar
Initial volume, V1 = 120 mL
Final volume, V2 = 180 mL
Since the temperature remains constant, the final pressure (p2) can be calculated using Boyle’s law.
According to Boyle’s law,
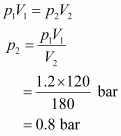
Therefore, the pressure would be 0.8 bar.
- Qstn #3Using the equation of state pV = nRT; show that at a given temperature density of a gas is proportional to gas pressurep.
Ans : The equation of state is given by,
pV = nRT ........... (i)
Where,
p → Pressure of gas
V → Volume of gas
n→ Number of moles of gas
R → Gas constant
T → Temperature of gas
From equation (i) we have,
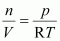
Replacing n with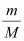 , we have
, we have
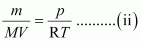
Where,
m → Mass of gas
M → Molar mass of gas
But,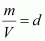 (d = density of gas)
(d = density of gas)
Thus, from equation (ii), we have
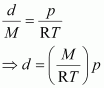
Molar mass (M) of a gas is always constant and therefore, at constant temperature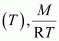 = constant.
= constant.
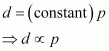
Hence, at a given temperature, the density (d) of gas is proportional to its pressure (p)
- Qstn #4At 0°C, the density of a certain oxide of a gas at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
Ans : Density (d) of the substance at temperature (T) can be given by the expression,
d =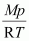
Now, density of oxide (d1) is given by,
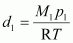
Where, M1 and p1 are the mass and pressure of the oxide respectively.
Density of dinitrogen gas (d2) is given by,
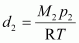
Where, M2 and p2 are the mass and pressure of the oxide respectively.
According to the given question,
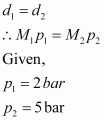
Molecular mass of nitrogen, M2 = 28 g/mol
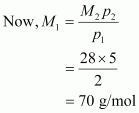
Hence, the molecular mass of the oxide is 70 g/mol.
- Qstn #5Pressure of 1 g of an ideal gas A at 27 °C is found to be 2 bar. When 2 g of another ideal gas B is introduced in the same flask at same temperature the pressure becomes 3 bar. Find a relationship between their molecular masses.
Ans : For ideal gas A, the ideal gas equation is given by,
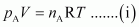
Where, pA and nA represent the pressure and number of moles of gas A.
For ideal gas B, the ideal gas equation is given by,
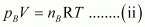
Where, pB and nB represent the pressure and number of moles of gas B.
[V and T are constants for gases A and B]
From equation (i), we have
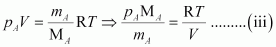
From equation (ii), we have
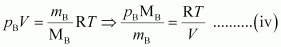
Where, MA and MB are the molecular masses of gases A and B respectively.
Now, from equations (iii) and (iv), we have
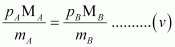
Given,
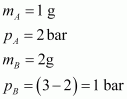
(Since total pressure is 3 bar)
Substituting these values in equation (v), we have
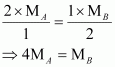
Thus, a relationship between the molecular masses of A and B is given by
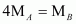 .
.
- Qstn #6The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20 °C and one bar will be released when 0.15g of aluminum reacts?
Ans : The reaction of aluminium with caustic soda can be represented as:
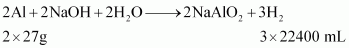
At STP (273.15 K and 1 atm), 54 g (2 × 27 g) of Al gives 3 × 22400 mL of H2..
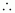 0.15 g Al gives
0.15 g Al gives 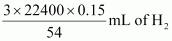 i.e., 186.67 mL of H2.
i.e., 186.67 mL of H2.
At STP,
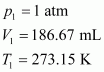
Let the volume of dihydrogen be at p2 = 0.987 atm (since 1 bar = 0.987 atm) and T2 = 20°C = (273.15 + 20) K = 293.15 K..
at p2 = 0.987 atm (since 1 bar = 0.987 atm) and T2 = 20°C = (273.15 + 20) K = 293.15 K..
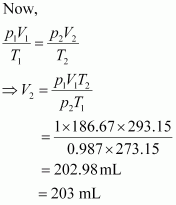
Therefore, 203 mL of dihydrogen will be released.
- Qstn #7What will be the pressure exerted by a mixture of 3.2 g of methane and 4.4 g of carbon dioxide contained in a 9 dm3 flask at 27 °C ?
Ans : It is known that,
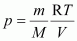
For methane (CH4),
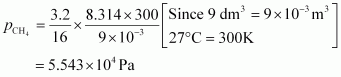
For carbon dioxide (CO2),
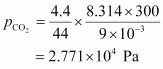
Total pressure exerted by the mixture can be obtained as:
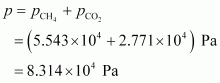
Hence, the total pressure exerted by the mixture is 8.314 × 104 Pa.
- Qstn #8What will be the pressure of the gaseous mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in a 1L vessel at 27°C?
Ans : Let the partial pressure of H2 in the vessel be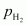 .
.
Now,
 = ?
= ?

It is known that,
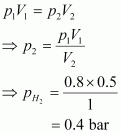
Now, let the partial pressure of O2 in the vessel be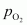 .
.
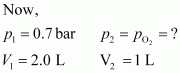
p1V1=p2V2⇒p2=p1V1V2⇒pO2=0.7×2.01=1.4bar
Total pressure of the gas mixture in the vessel can be obtained as:
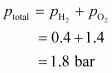
Hence, the total pressure of the gaseous mixture in the vessel is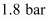 .
.
- Qstn #9Density of a gas is found to be 5.46 g/dm3 at 27 °C at 2 bar pressure. What will be its density at STP?
Ans : Given,
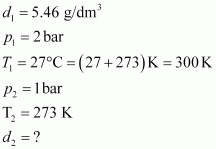
The density (d2) of the gas at STP can be calculated using the equation,
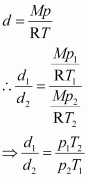
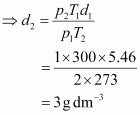
Hence, the density of the gas at STP will be 3 g dm-3.
- Qstn #1034.05 mL of phosphorus vapour weighs 0.0625 g at 546 °C and 0.1 bar pressure. What is the molar mass of phosphorus?
Ans : Given,
p = 0.1 bar
V = 34.05 mL = 34.05 × 10-3 L = 34.05 × 10-3 dm3
R = 0.083 bar dm3 K-1 mol-1
T = 546°C = (546 + 273) K = 819 K
The number of moles (n) can be calculated using the ideal gas equation as:
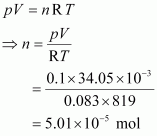
Therefore, molar mass of phosphorus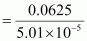 = 1247.5 g mol-1
= 1247.5 g mol-1
Hence, the molar mass of phosphorus is 1247.5 g mol-1.
- Qstn #11A student forgot to add the reaction mixture to the round bottomed flask at 27 °C but instead he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer he found the temperature of the flask was 477 °C. What fraction of air would have been expelled out?
Ans : Let the volume of the round bottomed flask be V.
Then, the volume of air inside the flask at 27° C is V.
Now,
V1 = V
T1 = 27°C = 300 K
V2 =?
T2 = 477° C = 750 K
According to Charles’s law,
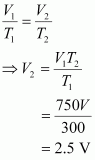
Therefore, volume of air expelled out = 2.5 V - V = 1.5 V
Hence, fraction of air expelled out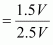
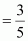
- Qstn #12Calculate the temperature of 4.0 mol of a gas occupying 5 dm3 at 3.32 bar.
(R = 0.083 bar dm3 K-1 mol-1).
Ans : Given,
n = 4.0 mol
V = 5 dm3
p = 3.32 bar
R = 0.083 bar dm3 K-1 mol-1
The temperature (T) can be calculated using the ideal gas equation as:
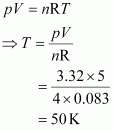
Hence, the required temperature is 50 K.
- Qstn #13Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Ans : Molar mass of dinitrogen (N2) = 28 g mol-1
Thus, 1.4 g of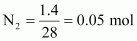
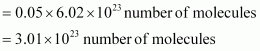
Now, 1 molecule of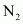 contains 14 electrons.
contains 14 electrons.
Therefore, 3.01 × 1023 molecules of N2 contains = 1.4 × 3.01 × 1023
= 4.214 × 1023 electrons
- Qstn #14How much time would it take to distribute one Avogadro number of wheat grains, if 1010 grains are distributed each second?
Ans : Avogadro number = 6.02 × 1023
Thus, time required
=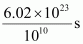
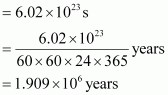
Hence, the time taken would be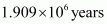 .
.