NEET-XI-Chemistry
05: States of Matter
- #13Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Ans : Molar mass of dinitrogen (N2) = 28 g mol-1
Thus, 1.4 g of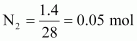
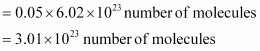
Now, 1 molecule of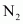 contains 14 electrons.
contains 14 electrons.
Therefore, 3.01 × 1023 molecules of N2 contains = 1.4 × 3.01 × 1023
= 4.214 × 1023 electrons