NEET-XI-Chemistry
05: States of Matter
- #7What will be the pressure exerted by a mixture of 3.2 g of methane and 4.4 g of carbon dioxide contained in a 9 dm3 flask at 27 °C ?
Ans : It is known that,
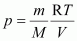
For methane (CH4),
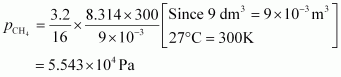
For carbon dioxide (CO2),
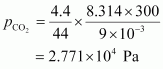
Total pressure exerted by the mixture can be obtained as:
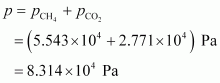
Hence, the total pressure exerted by the mixture is 8.314 × 104 Pa.