NEET-XI-Chemistry
05: States of Matter
- #8What will be the pressure of the gaseous mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in a 1L vessel at 27°C?
Ans : Let the partial pressure of H2 in the vessel be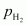 .
.
Now,
 = ?
= ?

It is known that,
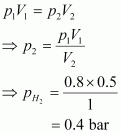
Now, let the partial pressure of O2 in the vessel be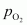 .
.
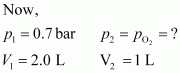
p1V1=p2V2⇒p2=p1V1V2⇒pO2=0.7×2.01=1.4bar
Total pressure of the gas mixture in the vessel can be obtained as:
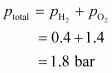
Hence, the total pressure of the gaseous mixture in the vessel is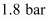 .
.