NEET-XI-Chemistry
05: States of Matter
- #1What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
Ans : Given,
Initial pressure, p1 = 1 bar
Initial volume, V1 = 500 dm3
Final volume, V2 = 200 dm3
Since the temperature remains constant, the final pressure (p2) can be calculated using Boyle’s law.
According to Boyle’s law,
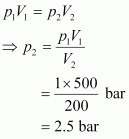
Therefore, the minimum pressure required is 2.5 bar.