NEET-XI-Chemistry
05: States of Matter
- #3Using the equation of state pV = nRT; show that at a given temperature density of a gas is proportional to gas pressurep.
Ans : The equation of state is given by,
pV = nRT ........... (i)
Where,
p → Pressure of gas
V → Volume of gas
n→ Number of moles of gas
R → Gas constant
T → Temperature of gas
From equation (i) we have,
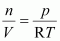
Replacing n with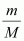 , we have
, we have
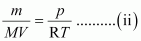
Where,
m → Mass of gas
M → Molar mass of gas
But,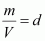 (d = density of gas)
(d = density of gas)
Thus, from equation (ii), we have
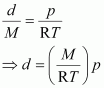
Molar mass (M) of a gas is always constant and therefore, at constant temperature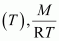 = constant.
= constant.
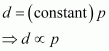
Hence, at a given temperature, the density (d) of gas is proportional to its pressure (p)