NEET-XI-Chemistry
05: States of Matter
- #6The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20 °C and one bar will be released when 0.15g of aluminum reacts?
Ans : The reaction of aluminium with caustic soda can be represented as:
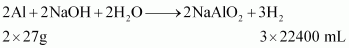
At STP (273.15 K and 1 atm), 54 g (2 × 27 g) of Al gives 3 × 22400 mL of H2..
 0.15 g Al gives
0.15 g Al gives 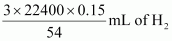 i.e., 186.67 mL of H2.
i.e., 186.67 mL of H2.
At STP,
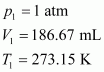
Let the volume of dihydrogen be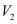 at p2 = 0.987 atm (since 1 bar = 0.987 atm) and T2 = 20°C = (273.15 + 20) K = 293.15 K..
at p2 = 0.987 atm (since 1 bar = 0.987 atm) and T2 = 20°C = (273.15 + 20) K = 293.15 K..
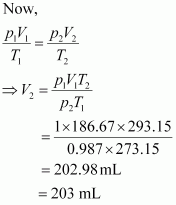
Therefore, 203 mL of dihydrogen will be released.