NEET-XI-Chemistry
05: States of Matter
- #5Pressure of 1 g of an ideal gas A at 27 °C is found to be 2 bar. When 2 g of another ideal gas B is introduced in the same flask at same temperature the pressure becomes 3 bar. Find a relationship between their molecular masses.
Ans : For ideal gas A, the ideal gas equation is given by,
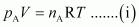
Where, pA and nA represent the pressure and number of moles of gas A.
For ideal gas B, the ideal gas equation is given by,
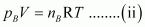
Where, pB and nB represent the pressure and number of moles of gas B.
[V and T are constants for gases A and B]
From equation (i), we have
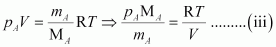
From equation (ii), we have
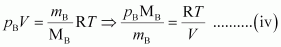
Where, MA and MB are the molecular masses of gases A and B respectively.
Now, from equations (iii) and (iv), we have
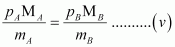
Given,
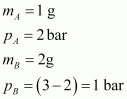
(Since total pressure is 3 bar)
Substituting these values in equation (v), we have
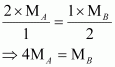
Thus, a relationship between the molecular masses of A and B is given by
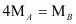 .
.