NEET-XI-Chemistry
05: States of Matter
- #1034.05 mL of phosphorus vapour weighs 0.0625 g at 546 °C and 0.1 bar pressure. What is the molar mass of phosphorus?
Ans : Given,
p = 0.1 bar
V = 34.05 mL = 34.05 × 10-3 L = 34.05 × 10-3 dm3
R = 0.083 bar dm3 K-1 mol-1
T = 546°C = (546 + 273) K = 819 K
The number of moles (n) can be calculated using the ideal gas equation as:
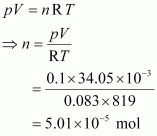
Therefore, molar mass of phosphorus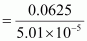 = 1247.5 g mol-1
= 1247.5 g mol-1
Hence, the molar mass of phosphorus is 1247.5 g mol-1.