NEET-XII-Chemistry
03: Electrochemistry
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #3 - Electrochemistry
- #Section : ISECTION I Page No 68:
- Qstn #1How would you determine the standard electrode potential of the systemMg2+ | Mg?
Ans : The standard electrode potential of Mg2+ | Mg can be measured with respect to the standard hydrogen electrode, represented by Pt(s), H2(g) (1 atm) | H+(aq)(1 M).
A cell, consisting of Mg | MgSO4 (aq 1 M) as the anode and the standard hydrogen electrode as the cathode, is set up.
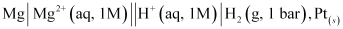
Then, the emf of the cell is measured and this measured emf is the standard electrode potential of the magnesium electrode.
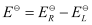
Here,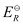 for the standard hydrogen electrode is zero.
for the standard hydrogen electrode is zero.
∴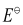
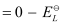

- Qstn #2Can you store copper sulphate solutions in a zinc pot?
Ans : Zinc is more reactive than copper. Therefore, zinc can displace copper from its salt solution. If copper sulphate solution is stored in a zinc pot, then zinc will displace copper from the copper sulphate solution.

Hence, copper sulphate solution cannot be stored in a zinc pot.
- Qstn #3Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions.
Ans : Substances that are stronger oxidising agents than ferrous ions can oxidise ferrous ions.
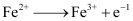 ;
; .gif) = -0.77 V
= -0.77 V
This implies that the substances having higher reduction potentials than +0.77 V can oxidise ferrous ions to ferric ions. Three substances that can do so are F2, Cl2, and O2.
- Qstn #4Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Ans : For hydrogen electrode,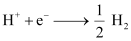 , it is given that pH = 10
, it is given that pH = 10
∴[H+] = 10-10 M
Now, using Nernst equation:
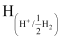 =
= 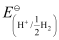
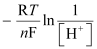
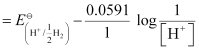
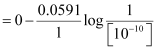
= -0.0591 log 1010
= -0.591 V
- Qstn #5Calculate the emf of the cell in which the following reaction takes place:
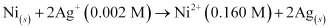
Given that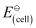 = 1.05 V
= 1.05 V
Ans : Applying Nernst equation we have:
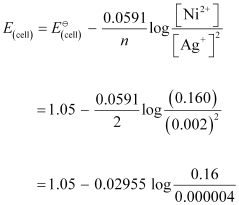
= 1.05 - 0.02955 log 4 × 104
= 1.05 - 0.02955 (log 10000 + log 4)
= 1.05 - 0.02955 (4 + 0.6021)
= 0.914 V
- Qstn #6The cell in which the following reactions occurs:

has
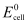 = 0.236 V at 298 K.
= 0.236 V at 298 K.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Ans : Here, n = 2,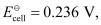 T = 298 K
T = 298 K
We know that:
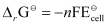
= -2 × 96487 × 0.236
= -45541.864 J mol-1
= -45.54 kJ mol-1
Again,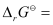 -2.303RT log Kc
-2.303RT log Kc
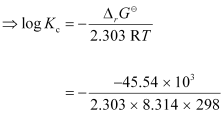
= 7.981
∴Kc = Antilog (7.981)
= 9.57 × 107
- Qstn #7Why does the conductivity of a solution decrease with dilution?
Ans : The conductivity of a solution is the conductance of ions present in a unit volume of the solution. The number of ions (responsible for carrying current) decreases when the solution is diluted. As a result, the conductivity of a solution decreases with dilution.
- Qstn #8Suggest a way to determine the
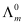 value of water.
value of water.
Ans : Applying Kohlrausch’s law of independent migration of ions, the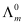 value of water can be determined as follows:
value of water can be determined as follows:
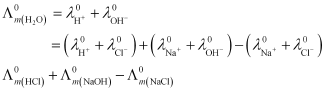
Hence, by knowing the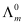 values of HCl, NaOH, and NaCl, the
values of HCl, NaOH, and NaCl, the 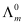 value of water can be determined.
value of water can be determined.
- Qstn #9The molar conductivity of 0.025 mol L-1 methanoic acid is
46.1 S cm2 mol-1.
Calculate its degree of dissociation and dissociation constant. Given λ °(H+)
= 349.6 S cm2 mol-1 and λ °(HCOO-) = 54.6 S cm2 mol
Ans : C = 0.025 mol L-1
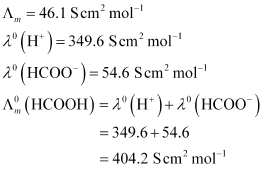
Now, degree of dissociation:

Thus, dissociation constant:
- Qstn #10If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Ans : I = 0.5 A
t = 2 hours = 2 × 60 × 60 s = 7200 s
Thus, Q = It
= 0.5 A × 7200 s
= 3600 C
We know that number of electrons.
number of electrons.
Then,
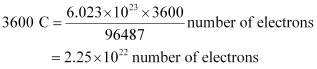
Hence,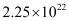 number of electrons will flow through the wire.
number of electrons will flow through the wire.
- Qstn #11Suggest a list of metals that are extracted electrolytically.
Ans : Metals that are on the top of the reactivity series such as sodium, potassium, calcium, lithium, magnesium, aluminium are extracted electrolytically.
- Qstn #12What is the quantity of electricity in coulombs needed to reduce 1 mol of
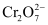 ? Consider the reaction:
? Consider the reaction:

Ans : The given reaction is as follows:
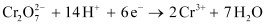
Therefore, to reduce 1 mole of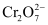 , the required quantity of electricity will be:
, the required quantity of electricity will be:
=6 F
= 6 × 96487 C
= 578922 C
- Qstn #13Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging.
Ans : A lead storage battery consists of a lead anode, a grid of lead packed with lead oxide (PbO2) as the cathode, and a 38% solution of sulphuric acid (H2SO4) as an electrolyte.
When the battery is in use, the following cell reactions take place:
At anode:
At cathode: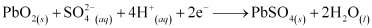
The overall cell reaction is given by,
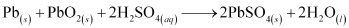
When a battery is charged, the reverse of all these reactions takes place.
Hence, on charging,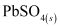 present at the anode and cathode is converted into
present at the anode and cathode is converted into 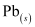 and
and 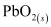 respectively.
respectively.