NEET-XII-Chemistry
03: Electrochemistry
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #14Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Ans : Methane and methanol can be used as fuels in fuel cells.
- Qstn #15Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
Ans : In the process of corrosion, due to the presence of air and moisture, oxidation takes place at a particular spot of an object made of iron. That spot behaves as the anode. The reaction at the anode is given by,
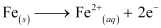
Electrons released at the anodic spot move through the metallic object and go to another spot of the object.
There, in the presence of H+ ions, the electrons reduce oxygen. This spot behaves as the cathode. These H+ ions come either from H2CO3, which are formed due to the dissolution of carbon dioxide from air into water or from the dissolution of other acidic oxides from the atmosphere in water.
The reaction corresponding at the cathode is given by,
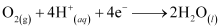
The overall reaction is:
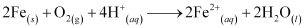
Also, ferrous ions are further oxidized by atmospheric oxygen to ferric ions. These ferric ions combine with moisture, present in the surroundings, to form hydrated ferric oxide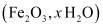 i.e., rust.
i.e., rust.
Hence, the rusting of iron is envisaged as the setting up of an electrochemical cell.
- #Section : IISECTION I Page No 92:
- Qstn #1Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Zn
Ans : The following is the order in which the given metals displace each other from the solution of their salts.
Mg, Al, Zn, Fe, Cu
- Qstn #2Given the standard electrode potentials,
K+/K = -2.93V, Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = -2.37 V, Cr3+/Cr = - 0.74V
Arrange these metals in their increasing order of reducing power.
Ans : The lower the reduction potential, the higher is the reducing power. The given standard electrode potentials increase in the order of K+/K < Mg2+/Mg < Cr3+/Cr < Hg2+/Hg < Ag+/Ag.
Hence, the reducing power of the given metals increases in the following order:
Ag < Hg < Cr < Mg < K
- Qstn #3Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place. Further show:
Ans : The galvanic cell in which the given reaction takes place is depicted as:
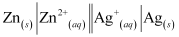
- Qstn #3-iWhich of the electrode is negatively charged?Ans : Zn electrode (anode) is negatively charged.
- Qstn #3-iiThe carriers of the current in the cell.Ans : Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
- Qstn #3-iiiIndividual reaction at each electrode.Ans : The reaction taking place at the anode is given by,
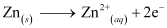
The reaction taking place at the cathode is given by,
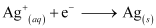
- Qstn #4Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
- Qstn #4-i2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3CdAns :
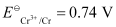
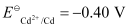
The galvanic cell of the given reaction is depicted as:
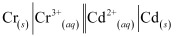
Now, the standard cell potential is
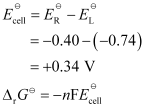
In the given equation,
n = 6
F = 96487 C mol-1
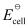 = +0.34 V
= +0.34 V
Then,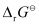 = -6 × 96487 C mol-1 × 0.34 V
= -6 × 96487 C mol-1 × 0.34 V
= -196833.48 CV mol-1
= -196833.48 J mol-1
= -196.83 kJ mol-1
Again,
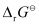 = -RT ln K
= -RT ln K
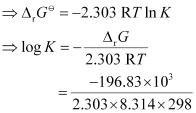
= 34.496
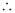 K = antilog (34.496)
K = antilog (34.496)
= 3.13 × 1034
- Qstn #4-iiFe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the ΔrGθ and equilibrium constant of the reactions.
Ans :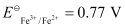
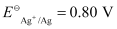
The galvanic cell of the given reaction is depicted as:
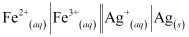
Now, the standard cell potential is
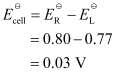
Here, n = 1.
Then,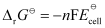
= -1 × 96487 C mol-1 × 0.03 V
= -2894.61 J mol-1
= -2.89 kJ mol-1
Again,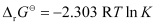
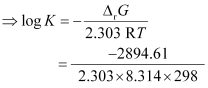
= 0.5073
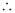 K = antilog (0.5073)
K = antilog (0.5073)
= 3.2 (approximately)
- Qstn #5-iMg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)Ans : For the given reaction, the Nernst equation can be given as:
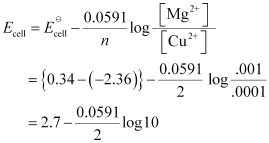
= 2.7 - 0.02955
= 2.67 V (approximately)
- Qstn #5-iiFe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)Ans : For the given reaction, the Nernst equation can be given as:
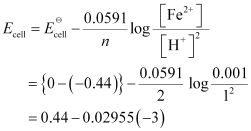
= 0.52865 V
= 0.53 V (approximately)