NEET-XII-Chemistry
03: Electrochemistry
- #10If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Ans : I = 0.5 A
t = 2 hours = 2 × 60 × 60 s = 7200 s
Thus, Q = It
= 0.5 A × 7200 s
= 3600 C
We know that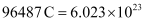 number of electrons.
number of electrons.
Then,
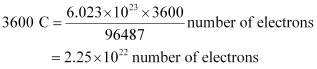
Hence,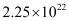 number of electrons will flow through the wire.
number of electrons will flow through the wire.