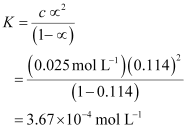NEET-XII-Chemistry
03: Electrochemistry
- #9The molar conductivity of 0.025 mol L-1 methanoic acid is
46.1 S cm2 mol-1.
Calculate its degree of dissociation and dissociation constant. Given λ °(H+)
= 349.6 S cm2 mol-1 and λ °(HCOO-) = 54.6 S cm2 mol
Ans : C = 0.025 mol L-1
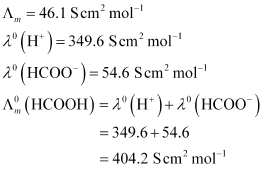
Now, degree of dissociation:

Thus, dissociation constant: