NEET-XII-Chemistry
03: Electrochemistry
- #6The cell in which the following reactions occurs:

has
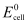 = 0.236 V at 298 K.
= 0.236 V at 298 K.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Ans : Here, n = 2,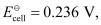 T = 298 K
T = 298 K
We know that:
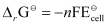
= -2 × 96487 × 0.236
= -45541.864 J mol-1
= -45.54 kJ mol-1
Again,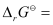 -2.303RT log Kc
-2.303RT log Kc
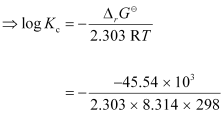
= 7.981
∴Kc = Antilog (7.981)
= 9.57 × 107