NEET-XII-Chemistry
03: Electrochemistry
- #3Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions.
Ans : Substances that are stronger oxidising agents than ferrous ions can oxidise ferrous ions.
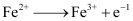 ;
; .gif) = -0.77 V
= -0.77 V
This implies that the substances having higher reduction potentials than +0.77 V can oxidise ferrous ions to ferric ions. Three substances that can do so are F2, Cl2, and O2.