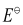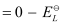NEET-XII-Chemistry
03: Electrochemistry
- #1How would you determine the standard electrode potential of the systemMg2+ | Mg?
Ans : The standard electrode potential of Mg2+ | Mg can be measured with respect to the standard hydrogen electrode, represented by Pt(s), H2(g) (1 atm) | H+(aq)(1 M).
A cell, consisting of Mg | MgSO4 (aq 1 M) as the anode and the standard hydrogen electrode as the cathode, is set up.
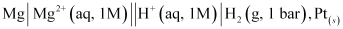
Then, the emf of the cell is measured and this measured emf is the standard electrode potential of the magnesium electrode.
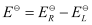
Here,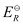 for the standard hydrogen electrode is zero.
for the standard hydrogen electrode is zero.
∴