NEET-XII-Chemistry
03: Electrochemistry
- #13Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging.
Ans : A lead storage battery consists of a lead anode, a grid of lead packed with lead oxide (PbO2) as the cathode, and a 38% solution of sulphuric acid (H2SO4) as an electrolyte.
When the battery is in use, the following cell reactions take place:
At anode:
At cathode: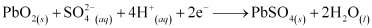
The overall cell reaction is given by,
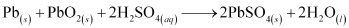
When a battery is charged, the reverse of all these reactions takes place.
Hence, on charging,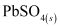 present at the anode and cathode is converted into
present at the anode and cathode is converted into 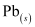 and
and 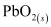 respectively.
respectively.