NEET-XII-Chemistry
02: Solutions
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #Section : APage No 37:
- Qstn #1Calculate
the mass percentage of benzene (C6H6)
and carbon tetrachloride (CCl4)
if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.Ans : Mass
percentage of C6H6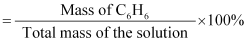
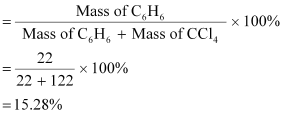
Mass
percentage of CCl4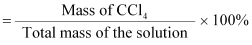
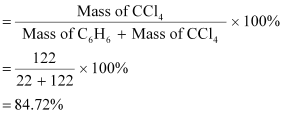
Alternatively,
Mass
percentage of CCl4 = (100 - 15.28)%
= 84.72%
- Qstn #2Calculate
the mole fraction of benzene in solution containing 30% by mass in
carbon tetrachloride.Ans : Let
the total mass of the solution be 100 g and the mass of benzene be 30
g.
∴Mass
of carbon tetrachloride = (100 - 30)g
=
70 g
Molar
mass of benzene (C6H6)
= (6 × 12 + 6 × 1) g mol-1
= 78
g mol-1
∴Number
of moles of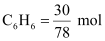
= 0.3846 mol
Molar
mass of carbon tetrachloride (CCl4)
= 1 × 12 + 4 × 355
=
154 g mol-1
∴Number
of moles of CCl4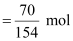
= 0.4545 mol
Thus,
the mole fraction of C6H6 is given as:
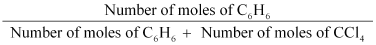
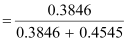
= 0.458
- #3-a30 g of Co(NO3)2.6H2O in 4 .3 L of solutionAns : Molar
mass of Co (NO3)2.6H2O
= 59 + 2 (14 + 3 × 16) + 6 × 18
= 291 g mol-1
∴Moles
of Co (NO3)2.6H2O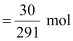
= 0.103 mol
Therefore,
molarity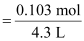
= 0.023 M
- #3-b30 mL of 0.5 M H2SO4 diluted to 500 mL.Ans : Number of moles present in 1000 mL of 0.5 M H2SO4 = 0.5 mol
∴Number
of moles present in 30 mL of 0.5 M H2SO4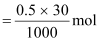
= 0.015 mol
Therefore, molarity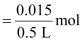
= 0.03 M
- Qstn #4Calculate the mass of urea (NH2CONH2)
required in making 2.5 kg of 0.25 molal aqueous solution.Ans : Molar
mass of urea (NH2CONH2)
= 2(1 × 14 + 2 × 1) + 1 × 12 + 1 × 16
= 60
g mol-1
0.25
molar aqueous solution of urea means:
1000 g of
water contains 0.25 mol = (0.25 × 60)g of urea
= 15 g of
urea
That is,
(1000
+ 15) g of solution contains 15 g of urea
Therefore,
2.5 kg (2500 g) of solution contains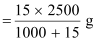
= 36.95 g
=
37 g of urea (approximately)
Hence,
mass of urea required = 37 g
Note: There is a slight variation in this answer and the one given in the
NCERT textbook.
- #5-amolalityAns : Molar
mass of KI = 39 + 127 = 166 g mol-1
20%
(mass/mass) aqueous solution of KI means 20
g of KI is present in 100 g of solution.
That is,
20
g of KI is present in (100 - 20) g of
water = 80 g of water
Therefore,
molality of the solution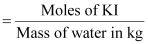
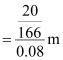
= 1.506 m
=
1.51 m (approximately)
- #5-bmolarity andAns : It
is given that the density of the solution = 1.202 g mL-1
∴Volume
of 100 g solution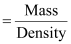
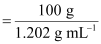
= 83.19 mL
=
83.19 × 10-3 L
Therefore,
molarity of the solution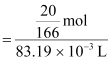
= 1.45 M
- #5-cmole fraction of KI if the density of 20% (mass/mass) aqueous KI is
1.202 g mL-1.Ans : Moles
of KI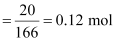
Moles
of water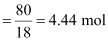
Therefore,
mole fraction of KI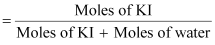
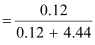
= 0.0263
Page No 41:
- Qstn #6H2S,
a toxic gas with rotten egg like smell, is used for the qualitative
analysis. If the solubility of H2S
in water at STP is 0.195 m, calculate Henry’s law constant.Ans : It
is given that the solubility of H2S
in water at STP is 0.195 m, i.e., 0.195 mol of H2S
is dissolved in 1000 g of water.
Moles
of water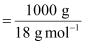
= 55.56
mol
∴Mole
fraction of H2S, x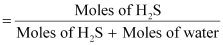
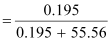
= 0.0035
At
STP, pressure (p)
= 0.987 bar
According
to Henry’s law:
p = KHx
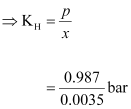
= 282
bar
- Qstn #7Henry’s
law constant for CO2 in water is 1.67 × 108 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.Ans : It
is given that:
KH = 1.67 × 108 Pa
 = 2.5 atm = 2.5 × 1.01325 × 105 Pa
= 2.5 atm = 2.5 × 1.01325 × 105 Pa
=
2.533125 × 105 Pa
According
to Henry’s law:
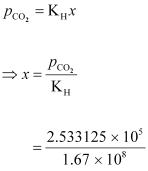
= 0.00152
We
can write,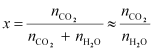
[Since, is
is
negligible as compared to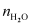 ]
]
In
500 mL of soda water, the volume of water =
500 mL
[Neglecting
the amount of soda present]
We can
write:
500 mL of
water = 500 g of water
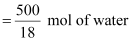
= 27.78 mol of water
Now,
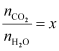
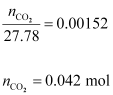
Hence,
quantity of CO2 in 500 mL of soda water = (0.042 × 44)g
= 1.848 g
Page No 47:
- Qstn #8The
vapour pressure of pure liquids A and B are 450 and 700 mm Hg
respectively, at 350 K. Find out the composition of the liquid
mixture if total vapour pressure is 600 mm Hg. Also find the
composition of the vapour phase.Ans : It is
given that:
 =
=
450 mm of Hg
 =
=
700 mm of Hg
ptotal = 600 mm of Hg
From
Raoult’s law, we have:
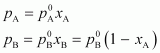
Therefore,
total pressure,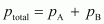
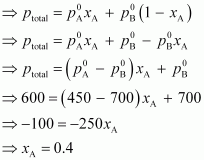
Therefore,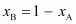
= 1 -
0.4
= 0.6
Now,
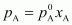
= 450 ×
0.4
= 180 mm
of Hg
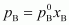
= 700 ×
0.6
= 420 mm
of Hg
Now, in
the vapour phase:
Mole
fraction of liquid A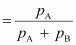
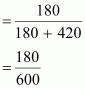
= 0.30
And,
mole fraction of liquid B = 1 - 0.30
=
0.70
Page No 55: