NEET-XII-Chemistry
02: Solutions
- #3Calculate
the molarity of each of the following solutions: (a) 30 g of Co(NO3)2.6H2O in 4 .3 L of solution (b) 30 mL of 0.5 M H2SO4 diluted to 500 mL.Ans : Molarity
is given by:

(a) Molar
mass of Co (NO3)2.6H2O
= 59 + 2 (14 + 3 × 16) + 6 × 18
= 291 g mol-1
∴Moles
of Co (NO3)2.6H2O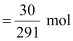
= 0.103 mol
Therefore,
molarity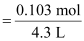
= 0.023 M (b) Number of moles present in 1000 mL of 0.5 M H2SO4 = 0.5 mol
∴Number
of moles present in 30 mL of 0.5 M H2SO4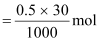
= 0.015 mol
Therefore, molarity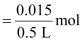
= 0.03 M
- #3-a30 g of Co(NO3)2.6H2O in 4 .3 L of solutionAns : Molar
mass of Co (NO3)2.6H2O
= 59 + 2 (14 + 3 × 16) + 6 × 18
= 291 g mol-1
∴Moles
of Co (NO3)2.6H2O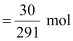
= 0.103 mol
Therefore,
molarity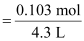
= 0.023 M
- #3-b30 mL of 0.5 M H2SO4 diluted to 500 mL.Ans : Number of moles present in 1000 mL of 0.5 M H2SO4 = 0.5 mol
∴Number
of moles present in 30 mL of 0.5 M H2SO4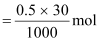
= 0.015 mol
Therefore, molarity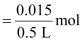
= 0.03 M