NEET-XII-Chemistry
02: Solutions
- #8The
vapour pressure of pure liquids A and B are 450 and 700 mm Hg
respectively, at 350 K. Find out the composition of the liquid
mixture if total vapour pressure is 600 mm Hg. Also find the
composition of the vapour phase.Ans : It is
given that:
 =
=
450 mm of Hg
 =
=
700 mm of Hg
ptotal = 600 mm of Hg
From
Raoult’s law, we have:
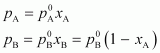
Therefore,
total pressure,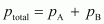
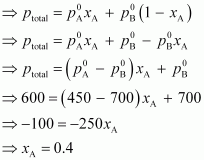
Therefore,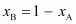
= 1 -
0.4
= 0.6
Now,
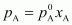
= 450 ×
0.4
= 180 mm
of Hg
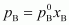
= 700 ×
0.6
= 420 mm
of Hg
Now, in
the vapour phase:
Mole
fraction of liquid A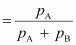
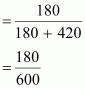
= 0.30
And,
mole fraction of liquid B = 1 - 0.30
=
0.70
Page No 55: