NEET-XII-Chemistry
02: Solutions
- #2Calculate
the mole fraction of benzene in solution containing 30% by mass in
carbon tetrachloride.Ans : Let
the total mass of the solution be 100 g and the mass of benzene be 30
g.
∴Mass
of carbon tetrachloride = (100 - 30)g
=
70 g
Molar
mass of benzene (C6H6)
= (6 × 12 + 6 × 1) g mol-1
= 78
g mol-1
∴Number
of moles of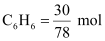
= 0.3846 mol
Molar
mass of carbon tetrachloride (CCl4)
= 1 × 12 + 4 × 355
=
154 g mol-1
∴Number
of moles of CCl4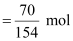
= 0.4545 mol
Thus,
the mole fraction of C6H6 is given as:
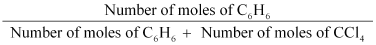
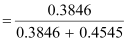
= 0.458