NEET-XII-Chemistry
02: Solutions
- #5Calculate (a) molality (b) molarity and (c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI is
1.202 g mL-1. (a) molality (b) molarity and (c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI is
1.202 g mL-1.Ans : (a) Molar
mass of KI = 39 + 127 = 166 g mol-1
20%
(mass/mass) aqueous solution of KI means 20
g of KI is present in 100 g of solution.
That is,
20
g of KI is present in (100 - 20) g of
water = 80 g of water
Therefore,
molality of the solution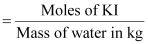
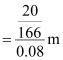
= 1.506 m
=
1.51 m (approximately)
(b) It
is given that the density of the solution = 1.202 g mL-1
∴Volume
of 100 g solution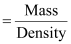
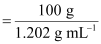
= 83.19 mL
=
83.19 × 10-3 L
Therefore,
molarity of the solution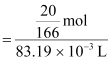
= 1.45 M
(c) Moles
of KI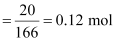
Moles
of water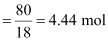
Therefore,
mole fraction of KI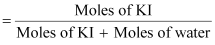
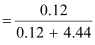
= 0.0263
Page No 41: (a) Molar
mass of KI = 39 + 127 = 166 g mol-1
20%
(mass/mass) aqueous solution of KI means 20
g of KI is present in 100 g of solution.
That is,
20
g of KI is present in (100 - 20) g of
water = 80 g of water
Therefore,
molality of the solution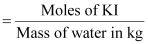
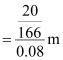
= 1.506 m
=
1.51 m (approximately)
(b) It
is given that the density of the solution = 1.202 g mL-1
∴Volume
of 100 g solution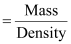
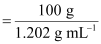
= 83.19 mL
=
83.19 × 10-3 L
Therefore,
molarity of the solution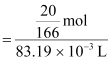
= 1.45 M
(c) Moles
of KI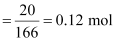
Moles
of water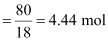
Therefore,
mole fraction of KI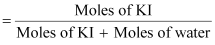
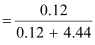
= 0.0263
Page No 41:
- #5-amolalityAns : Molar
mass of KI = 39 + 127 = 166 g mol-1
20%
(mass/mass) aqueous solution of KI means 20
g of KI is present in 100 g of solution.
That is,
20
g of KI is present in (100 - 20) g of
water = 80 g of water
Therefore,
molality of the solution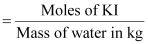
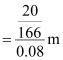
= 1.506 m
=
1.51 m (approximately)
- #5-bmolarity andAns : It
is given that the density of the solution = 1.202 g mL-1
∴Volume
of 100 g solution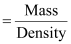
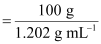
= 83.19 mL
=
83.19 × 10-3 L
Therefore,
molarity of the solution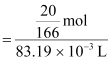
= 1.45 M
- #5-cmole fraction of KI if the density of 20% (mass/mass) aqueous KI is
1.202 g mL-1.Ans : Moles
of KI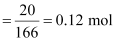
Moles
of water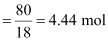
Therefore,
mole fraction of KI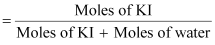
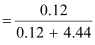
= 0.0263
Page No 41: