NEET-XII-Chemistry
02: Solutions
- #4Calculate the mass of urea (NH2CONH2)
required in making 2.5 kg of 0.25 molal aqueous solution.Ans : Molar
mass of urea (NH2CONH2)
= 2(1 × 14 + 2 × 1) + 1 × 12 + 1 × 16
= 60
g mol-1
0.25
molar aqueous solution of urea means:
1000 g of
water contains 0.25 mol = (0.25 × 60)g of urea
= 15 g of
urea
That is,
(1000
+ 15) g of solution contains 15 g of urea
Therefore,
2.5 kg (2500 g) of solution contains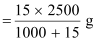
= 36.95 g
=
37 g of urea (approximately)
Hence,
mass of urea required = 37 g
Note: There is a slight variation in this answer and the one given in the
NCERT textbook.