NEET-XI-Chemistry
06: Chemical Thermodynamics
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- #Chapter 6 - Chemical Thermodynamics
- Qstn #1Choose the correct answer. A thermodynamic state function is a quantity
(i) used to determine heat changes
(ii) whose value is independent of path
(iii) used to determine pressure volume work
(iv) whose value depends on temperature only.Ans : A thermodynamic state function is a quantity whose value is independent of a path.
Functions like p, V, T etc. depend only on the state of a system and not on the path.
Hence, alternative (ii) is correct.
- Qstn #2For the process to occur under adiabatic conditions, the correct condition is:
(i) ΔT = 0
(ii) Δp = 0
(iii) q = 0
(iv) w = 0Ans : A system is said to be under adiabatic conditions if there is no exchange of heat between the system and its surroundings. Hence, under adiabatic conditions, q = 0.
Therefore, alternative (iii) is correct.
- Qstn #3The enthalpies of all elements in their standard states are:
(i) unity
(ii) zero
(iii) < 0
(iv) different for each elementAns : The enthalpy of all elements in their standard state is zero.
Therefore, alternative (ii) is correct.
- Qstn #4ΔUθof combustion of methane is - X kJ mol-1. The value of ΔHθ is
(i) = ΔUθ
(ii) > ΔUθ
(iii) < ΔUθ
(iv) = 0Ans : Since ΔHθ = ΔUθ + ΔngRT and ΔUθ = -X kJ mol-1,
ΔHθ = (-X) + ΔngRT.
⇒ ΔHθ < ΔUθ
Therefore, alternative (iii) is correct.
- Qstn #5The enthalpy of combustion of methane, graphite and dihydrogen at 298 K are, -890.3 kJ mol-1 -393.5 kJ mol-1, and -285.8 kJ mol-1 respectively. Enthalpy of formation of CH4(g) will be
(i) -74.8 kJ mol-1 (ii) -52.27 kJ mol-1
(iii) +74.8 kJ mol-1 (iv) +52.26 kJ mol-1.
Ans : According to the question,
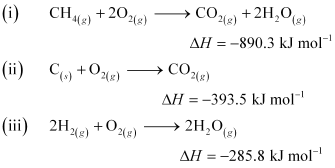
Thus, the desired equation is the one that represents the formation of CH4 (g) i.e.,
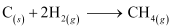

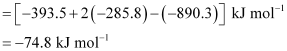
 Enthalpy of formation of CH4(g) = -74.8 kJ mol-1
Enthalpy of formation of CH4(g) = -74.8 kJ mol-1
Hence, alternative (i) is correct.
- Qstn #6A reaction, A + B → C + D + q is found to have a positive entropy change. The reaction will be
(i) possible at high temperature
(ii) possible only at low temperature
(iii) not possible at any temperature
(iv) possible at any temperatureAns : For a reaction to be spontaneous, ΔG should be negative.
ΔG = ΔH - TΔS
According to the question, for the given reaction,
ΔS = positive
ΔH = negative (since heat is evolved)
⇒ ΔG = negative
Therefore, the reaction is spontaneous at any temperature.
Hence, alternative (iv) is correct.
- Qstn #7In a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process?
Ans : According to the first law of thermodynamics,
ΔU = q + W (i)
Where,
ΔU = change in internal energy for a process
q = heat
W = work
Given,
q = + 701 J (Since heat is absorbed)
W = -394 J (Since work is done by the system)
Substituting the values in expression (i), we get
ΔU = 701 J + (-394 J)
ΔU = 307 J
Hence, the change in internal energy for the given process is 307 J.
- Qstn #8The reaction of cyanamide, NH2CN(s),with dioxygen was carried out in a bomb calorimeter, and ΔU was found to be –742.7 kJ mol–1at 298 K. Calculate enthalpy change for the reaction at 298 K.
$$\ce{NH2CN(s) + \frac32 O2(g) -> N2(g) + CO2(g) + H2O(l) } $$Ans : Enthalpy change for a reaction (ΔH) is given by the expression,
ΔH = ΔU + ΔngRT
Where,
ΔU = change in internal energy
Δng = change in number of moles
For the given reaction,
Δng = ∑ng (products) – ∑ng (reactants)
= (2 – 1.5) moles
Δng = 0.5 moles
And,
ΔU = –742.7 kJ mol–1
T = 298 K
R = 8.314 × 10–3 kJ mol–1 K–1
Substituting the values in the expression of ΔH:
ΔH = (–742.7 kJ mol–1) + (0.5 mol) (298 K) (8.314 × 10–3 kJ mol–1 K–1)
= –742.7 + 1.2
ΔH = –741.5 kJ mol–1
- Qstn #9Calculate the number of kJ of heat necessary to raise the temperature of 60.0 g of aluminium from 35°C to 55°C. Molar heat capacity of Al is 24 J mol-1 K-1.
Ans : From the expression of heat (q),
q = m. c. ΔT
Where,
c = molar heat capacity
m = mass of substance
ΔT = change in temperature
Substituting the values in the expression of q:
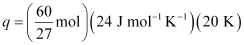
q = 1066.7 J
q = 1.07 kJ
- Qstn #10Calculate the enthalpy change on freezing of 1.0 mol of water at 10.0°C to ice at -10.0°C. ΔfusH = 6.03 kJ mol-1 at 0°C.
Cp[H2O(l)] = 75.3 J mol-1 K-1
Cp[H2O(s)] = 36.8 J mol-1 K-1
Ans : Total enthalpy change involved in the transformation is the sum of the following changes:
(a) Energy change involved in the transformation of 1 mol of water at 10°C to 1 mol of water at 0°C.
(b) Energy change involved in the transformation of 1 mol of water at 0° to 1 mol of ice at 0°C.
(c) Energy change involved in the transformation of 1 mol of ice at 0°C to 1 mol of ice at -10°C.
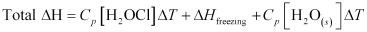
= (75.3 J mol-1 K-1) (0 - 10)K + (-6.03 × 103 J mol-1) + (36.8 J mol-1 K-1) (-10 - 0)K
= -753 J mol-1 - 6030 J mol-1 - 368 J mol-1
= -7151 J mol-1
= -7.151 kJ mol-1
Hence, the enthalpy change involved in the transformation is -7.151 kJ mol-1.
- Qstn #11Enthalpy of combustion of carbon to CO2 is -393.5 kJ mol-1. Calculate the heat released upon formation of 35.2 g of CO2 from carbon and dioxygen gas.
Ans : Formation of CO2 from carbon and dioxygen gas can be represented as:
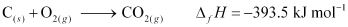
(1 mole = 44 g)
Heat released on formation of 44 g CO2 = -393.5 kJ mol-1
.gif) Heat released on formation of 35.2 g CO2
Heat released on formation of 35.2 g CO2

= -314.8 kJ mol-1
- Qstn #12Enthalpies of formation of CO(g), CO2(g), N2O(g) and N2O4(g) are -110 kJ mol-1, - 393 kJ mol-1, 81 kJ mol-1 and 9.7 kJ mol-1 respectively. Find the value of ΔrH for the reaction:
N2O4(g) + 3CO(g)
 N2O(g) + 3CO2(g)
N2O(g) + 3CO2(g)
Ans : ΔrH for a reaction is defined as the difference between ΔfH value of products and ΔfH value of reactants.
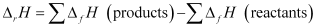
For the given reaction,
N2O4(g) + 3CO(g)
 N2O(g) + 3CO2(g)
N2O(g) + 3CO2(g)

Substituting the values of ΔfH for N2O, CO2, N2O4, and CO from the question, we get:
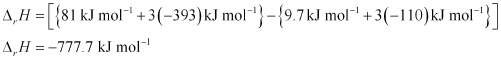
Hence, the value of ΔrH for the reaction is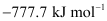 .
.
- Qstn #13Given
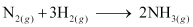 ; ΔrHθ = -92.4 kJ mol-1
; ΔrHθ = -92.4 kJ mol-1
What is the standard enthalpy of formation of NH3 gas?
Ans : Standard enthalpy of formation of a compound is the change in enthalpy that takes place during the formation of 1 mole of a substance in its standard form from its constituent elements in their standard state.
Re-writing the given equation for 1 mole of NH3(g),
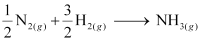
.gif) Standard enthalpy of formation of NH3(g)
Standard enthalpy of formation of NH3(g)
= ½ ΔrHθ
= ½ (-92.4 kJ mol-1)
= -46.2 kJ mol-1
- Qstn #14Calculate the standard enthalpy of formation of CH3OH(l) from the following data:
CH3OH(l) +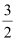 O2(g)
O2(g) .gif) CO2(g) + 2H2O(l) ; ΔrHθ = -726 kJ mol-1
CO2(g) + 2H2O(l) ; ΔrHθ = -726 kJ mol-1
C(g) + O2(g).gif) CO2(g) ; ΔcHθ = -393 kJ mol-1
CO2(g) ; ΔcHθ = -393 kJ mol-1
H2(g) +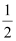 O2(g)
O2(g) .gif) H2O(l) ; ΔfHθ = -286 kJ mol-1.
H2O(l) ; ΔfHθ = -286 kJ mol-1.
Ans : The reaction that takes place during the formation of CH3OH(l) can be written as:
C(s) + 2H2O(g) +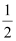 O2(g)
O2(g)
.gif) CH3OH(l) (1)
CH3OH(l) (1)
The reaction (1) can be obtained from the given reactions by following the algebraic calculations as:
Equation (ii) + 2 × equation (iii) - equation (i)
ΔfHθ [CH3OH(l)] = ΔcHθ + 2ΔfHθ [H2O(l)] - ΔrHθ
= (-393 kJ mol-1) + 2(-286 kJ mol-1) - (-726 kJ mol-1)
= (-393 - 572 + 726) kJ mol-1
.gif) ΔfHθ [CH3OH(l)] = -239 kJ mol-1
ΔfHθ [CH3OH(l)] = -239 kJ mol-1