NEET-XI-Chemistry
06: Chemical Thermodynamics
- #14Calculate the standard enthalpy of formation of CH3OH(l) from the following data:
CH3OH(l) +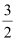 O2(g)
O2(g) .gif) CO2(g) + 2H2O(l) ; ΔrHθ = -726 kJ mol-1
CO2(g) + 2H2O(l) ; ΔrHθ = -726 kJ mol-1
C(g) + O2(g).gif) CO2(g) ; ΔcHθ = -393 kJ mol-1
CO2(g) ; ΔcHθ = -393 kJ mol-1
H2(g) +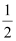 O2(g)
O2(g) .gif) H2O(l) ; ΔfHθ = -286 kJ mol-1.
H2O(l) ; ΔfHθ = -286 kJ mol-1.
Ans : The reaction that takes place during the formation of CH3OH(l) can be written as:
C(s) + 2H2O(g) +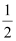 O2(g)
O2(g)
.gif) CH3OH(l) (1)
CH3OH(l) (1)
The reaction (1) can be obtained from the given reactions by following the algebraic calculations as:
Equation (ii) + 2 × equation (iii) - equation (i)
ΔfHθ [CH3OH(l)] = ΔcHθ + 2ΔfHθ [H2O(l)] - ΔrHθ
= (-393 kJ mol-1) + 2(-286 kJ mol-1) - (-726 kJ mol-1)
= (-393 - 572 + 726) kJ mol-1
.gif) ΔfHθ [CH3OH(l)] = -239 kJ mol-1
ΔfHθ [CH3OH(l)] = -239 kJ mol-1