NEET-XI-Chemistry
06: Chemical Thermodynamics
- #10Calculate the enthalpy change on freezing of 1.0 mol of water at 10.0°C to ice at -10.0°C. ΔfusH = 6.03 kJ mol-1 at 0°C.
Cp[H2O(l)] = 75.3 J mol-1 K-1
Cp[H2O(s)] = 36.8 J mol-1 K-1
Ans : Total enthalpy change involved in the transformation is the sum of the following changes:
(a) Energy change involved in the transformation of 1 mol of water at 10°C to 1 mol of water at 0°C.
(b) Energy change involved in the transformation of 1 mol of water at 0° to 1 mol of ice at 0°C.
(c) Energy change involved in the transformation of 1 mol of ice at 0°C to 1 mol of ice at -10°C.
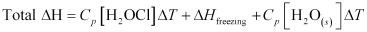
= (75.3 J mol-1 K-1) (0 - 10)K + (-6.03 × 103 J mol-1) + (36.8 J mol-1 K-1) (-10 - 0)K
= -753 J mol-1 - 6030 J mol-1 - 368 J mol-1
= -7151 J mol-1
= -7.151 kJ mol-1
Hence, the enthalpy change involved in the transformation is -7.151 kJ mol-1.