NEET-XI-Chemistry
06: Chemical Thermodynamics
- #9Calculate the number of kJ of heat necessary to raise the temperature of 60.0 g of aluminium from 35°C to 55°C. Molar heat capacity of Al is 24 J mol-1 K-1.
Ans : From the expression of heat (q),
q = m. c. ΔT
Where,
c = molar heat capacity
m = mass of substance
ΔT = change in temperature
Substituting the values in the expression of q:
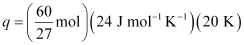
q = 1066.7 J
q = 1.07 kJ