NEET-XI-Chemistry
06: Chemical Thermodynamics
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Qstn #15Calculate the enthalpy change for the process
CCl4(g) → C(g) + 4Cl(g)
and calculate bond enthalpy of C-Cl in CCl4(g).
ΔvapHθ (CCl4) = 30.5 kJ mol-1.
ΔfHθ (CCl4) = -135.5 kJ mol-1.
ΔaHθ (C) = 715.0 kJ mol-1, where ΔaHθ is enthalpy of atomisation
ΔaHθ (Cl2) = 242 kJ mol-1
Ans : The chemical equations implying to the given values of enthalpies are:
 ΔvapHθ = 30.5 kJ mol-1
ΔvapHθ = 30.5 kJ mol-1
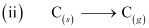 ΔaHθ = 715.0 kJ mol-1
ΔaHθ = 715.0 kJ mol-1
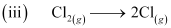 ΔaHθ = 242 kJ mol-1
ΔaHθ = 242 kJ mol-1
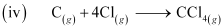 ΔfH = -135.5 kJ mol-1
ΔfH = -135.5 kJ mol-1
Enthalpy change for the given process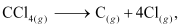 can be calculated using the following algebraic calculations as:
can be calculated using the following algebraic calculations as:
Equation (ii) + 2 × Equation (iii) - Equation (i) - Equation (iv)
ΔH = ΔaHθ(C) + 2ΔaHθ (Cl2) - ΔvapHθ - ΔfH
= (715.0 kJ mol-1) + 2(242 kJ mol-1) - (30.5 kJ mol-1) - (-135.5 kJ mol-1)
.gif) ΔH = 1304 kJ mol-1
ΔH = 1304 kJ mol-1
Bond enthalpy of C-Cl bond in CCl4 (g)
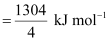
= 326 kJ mol-1
- Qstn #16For an isolated system, ΔU = 0, what will be ΔS?
Ans : ΔS will be positive i.e., greater than zero
Since ΔU = 0, ΔS will be positive and the reaction will be spontaneous.
- Qstn #17For the reaction at 298 K,
2A + B → C
ΔH = 400 kJ mol-1 and ΔS = 0.2 kJ K-1 mol-1
At what temperature will the reaction become spontaneous considering ΔH and ΔS to be constant over the temperature range?
Ans : From the expression,
ΔG = ΔH - TΔS
Assuming the reaction at equilibrium, ΔT for the reaction would be:
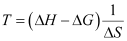
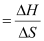 (ΔG = 0 at equilibrium)
(ΔG = 0 at equilibrium)
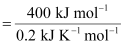
T = 2000 K
For the reaction to be spontaneous, ΔG must be negative. Hence, for the given reaction to be spontaneous, T should be greater than 2000 K.
- Qstn #18For the reaction,
2Cl(g) → Cl2(g), what are the signs of ΔH and ΔS ?
Ans : ΔH and ΔS are negative
The given reaction represents the formation of chlorine molecule from chlorine atoms. Here, bond formation is taking place. Therefore, energy is being released. Hence, ΔH is negative.
Also, two moles of atoms have more randomness than one mole of a molecule. Since spontaneity is decreased, ΔS is negative for the given reaction.
- Qstn #19For the reaction
2A(g) + B(g) → 2D(g)
ΔUθ = -10.5 kJ and ΔSθ= -44.1 JK-1.
Calculate ΔGθ for the reaction, and predict whether the reaction may occur spontaneously.
Ans : For the given reaction,
2 A(g) + B(g) → 2D(g)
Δng = 2 - (3)
= -1 mole
Substituting the value of ΔUθ in the expression of ΔH:
ΔHθ = ΔUθ + ΔngRT
= (-10.5 kJ) - (-1) (8.314 × 10-3 kJ K-1 mol-1) (298 K)
= -10.5 kJ - 2.48 kJ
ΔHθ = -12.98 kJ
Substituting the values of ΔHθ and ΔSθ in the expression of ΔGθ:
ΔGθ = ΔHθ - TΔSθ
= -12.98 kJ - (298 K) (-44.1 J K-1)
= -12.98 kJ + 13.14 kJ
ΔGθ = + 0.16 kJ
Since ΔGθ for the reaction is positive, the reaction will not occur spontaneously.
- Qstn #20The equilibrium constant for a reaction is 10. What will be the value of ΔGθ? R = 8.314 JK-1 mol-1, T = 300 K.
Ans : From the expression,
ΔGθ = -2.303 RT logKeq
ΔGθ for the reaction,
= (2.303) (8.314 JK-1 mol-1) (300 K) log10
= -5744.14 Jmol-1
= -5.744 kJ mol-1
- Qstn #21Comment on the thermodynamic stability of NO(g), given
.gif) N2(g) +
N2(g) + .gif) O2(g) → NO(g) ; ΔrHθ = 90 kJ mol-1
O2(g) → NO(g) ; ΔrHθ = 90 kJ mol-1
NO(g) +.gif) O2(g) → NO2(g) : ΔrHθ= -74 kJ mol-1
O2(g) → NO2(g) : ΔrHθ= -74 kJ mol-1
Ans : The positive value of ΔrH indicates that heat is absorbed during the formation of NO(g). This means that NO(g) has higher energy than the reactants (N2 and O2). Hence, NO(g) is unstable.
The negative value of ΔrH indicates that heat is evolved during the formation of NO2(g) from NO(g) and O2(g). The product, NO2(g) is stabilized with minimum energy.
Hence, unstable NO(g) changes to stable NO2(g).
- Qstn #22Calculate the entropy change in surroundings when 1.00 mol of H2O(l) is formed under standard conditions. ΔfHθ = -286 kJ mol-1.
Ans : It is given that 286 kJ mol-1 of heat is evolved on the formation of 1 mol of H2O(l). Thus, an equal amount of heat will be absorbed by the surroundings.
qsurr = +286 kJ mol-1
Entropy change (ΔSsurr) for the surroundings =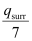

.gif) ΔSsurr = 959.73 J mol-1 K-1
ΔSsurr = 959.73 J mol-1 K-1