NEET-XI-Chemistry
06: Chemical Thermodynamics
- #11Enthalpy of combustion of carbon to CO2 is -393.5 kJ mol-1. Calculate the heat released upon formation of 35.2 g of CO2 from carbon and dioxygen gas.
Ans : Formation of CO2 from carbon and dioxygen gas can be represented as:
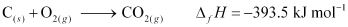
(1 mole = 44 g)
Heat released on formation of 44 g CO2 = -393.5 kJ mol-1
.gif) Heat released on formation of 35.2 g CO2
Heat released on formation of 35.2 g CO2

= -314.8 kJ mol-1