NEET-XII-Chemistry
03: Electrochemistry
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #5-iiiSn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)Ans : For the given reaction, the Nernst equation can be given as:
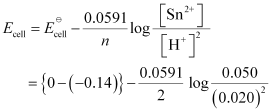
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
- Qstn #5-ivPt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).Ans : For the given reaction, the Nernst equation can be given as:
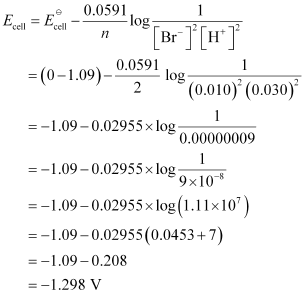
- Qstn #6In the button cells widely used in watches and other devices the following reaction takes place:
Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH-(aq)
Determine
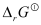 and
and 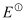 for the reaction.
for the reaction.
Ans :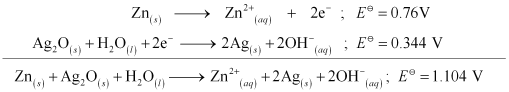
.gif)
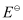 = 1.104 V
= 1.104 V
We know that,
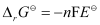
= -2 × 96487 × 1.04
= -213043.296 J
= -213.04 kJ
- Qstn #7Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Ans : Conductivity of a solution is defined as the conductance of a solution of 1 cm in length and area of cross-section 1 sq. cm. The inverse of resistivity is called conductivity or specific conductance. It is represented by the symbolκ. If ``\rho`` is resistivity, then we can write:

The conductivity of a solution at any given concentration is the conductance (G) of one unit volume of solution kept between two platinum electrodes with the unit area of cross-section and at a distance of unit length.
i.e.,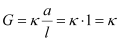
(Since a = 1, l = 1)
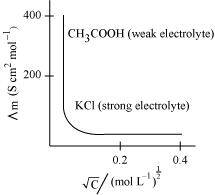
Conductivity always decreases with a decrease in concentration, both for weak and strong electrolytes. This is because the number of ions per unit volume that carry the current in a solution decreases with a decrease in concentration.
Molar conductivity:
Molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.
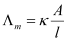
Now, l = 1 and A = V (volume containing 1 mole of the electrolyte).
.gif)
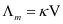
Molar conductivity increases with a decrease in concentration. This is because the total volume V of the solution containing one mole of the electrolyte increases on dilution.
The variation of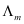 with
with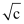 for strong and weak electrolytes is shown in the following plot:
for strong and weak electrolytes is shown in the following plot:
- Qstn #8The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 Scm-1. Calculate its molar conductivity.
Ans : Given,
κ = 0.0248 S cm-1
c = 0.20 M
.gif) Molar conductivity,
Molar conductivity, 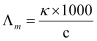
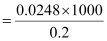
= 124 Scm2mol-1
- Qstn #9The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 ``\Omega``. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1.
Ans : Given,
Conductivity, κ = 0.146 × 10-3 S cm-1
Resistance, R = 1500 ``\Omega``
.gif) Cell constant = κ × R
Cell constant = κ × R
= 0.146 × 10-3 × 1500
= 0.219 cm-1
- Qstn #10The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
Concentration/M 0.001 0.010 0.020 0.050 0.100
102 × κ/S m-1 1.237 11.85 23.15 55.53 106.74
Calculate.gif) for all concentrations and draw a plot between
for all concentrations and draw a plot between  and c½. Find the value of
and c½. Find the value of .
.
Ans : Given,
κ = 1.237 × 10-2 S m-1, c = 0.001 M
Then, κ = 1.237 × 10-4 S cm-1, c½ = 0.0316 M1/2
.gif)
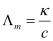
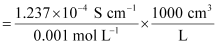
= 123.7 S cm2 mol-1
Given,
κ = 11.85 × 10-2 S m-1, c = 0.010M
Then, κ = 11.85 × 10-4 S cm-1, c½ = 0.1 M1/2
.gif)
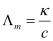
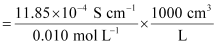
= 118.5 S cm2 mol-1
Given,
κ = 23.15 × 10-2 S m-1, c = 0.020 M
Then, κ = 23.15 × 10-4 S cm-1, c1/2 = 0.1414 M1/2
.gif)
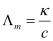
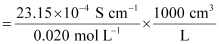
= 115.8 S cm2 mol-1
Given,
κ = 55.53 × 10-2 S m-1, c = 0.050 M
Then, κ = 55.53 × 10-4 S cm-1, c1/2 = 0.2236 M1/2
.gif)
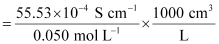
= 111.1 1 S cm2 mol-1
Given,
κ = 106.74 × 10-2 S m-1, c = 0.100 M
Then, κ = 106.74 × 10-4 S cm-1, c1/2 = 0.3162 M1/2
.gif)
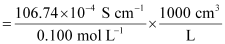
= 106.74 S cm2 mol-1
Now, we have the following data:
-
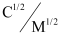
0.0316
0.1
0.1414
0.2236
0.3162
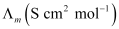
123.7
118.5
115.8
111.1
106.74
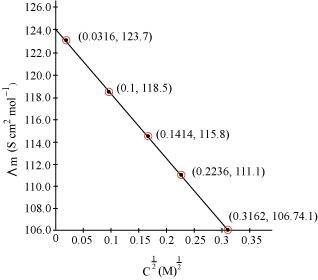
Since the line interrupts.gif) at 124.0 S cm2 mol-1,
at 124.0 S cm2 mol-1,  = 124.0 S cm2 mol-1.
= 124.0 S cm2 mol-1.
-
- Qstn #11Conductivity of 0.00241 M acetic acid is 7.896 × 10-5 S cm-1. Calculate its molar conductivity and if
.gif) for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant?
for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant?
Ans : Given, κ = 7.896 × 10-5 S m-1
c = 0.00241 mol L-1
Then, molar conductivity,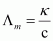
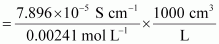
= 32.76S cm2 mol-1
Again,.gif) = 390.5 S cm2 mol-1
= 390.5 S cm2 mol-1
Now,
= 0.084
.gif) Dissociation constant,
Dissociation constant, 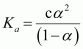
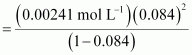
= 1.86 × 10-5 mol L-1
- Qstn #13-i20.0 g of Ca from molten CaCl2.Ans : According to the question,
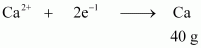
Electricity required to produce 40 g of calcium = 2 F
Therefore, electricity required to produce 20 g of calcium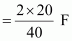
= 1 F
- Qstn #13-ii40.0 g of Al from molten Al2O3.Ans : According to the question,
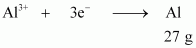
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al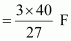
= 4.44 F