NEET-XII-Chemistry
03: Electrochemistry
- #13How much electricity in terms of Faraday is required to produce
() 20.0 g of Ca from molten CaCl2.
() 40.0 g of Al from molten Al2O3.
() 40.0 g of Al from molten Al2O3.
() 40.0 g of Al from molten Al2O3.
() 20.0 g of Ca from molten CaCl2.
() 40.0 g of Al from molten Al2O3.
() 40.0 g of Al from molten Al2O3.
() 40.0 g of Al from molten Al2O3.Ans : null () According to the question,
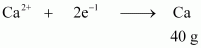
Electricity required to produce 40 g of calcium = 2 F
Therefore, electricity required to produce 20 g of calcium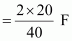
= 1 F
() According to the question,
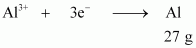
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al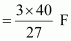
= 4.44 F
() According to the question,
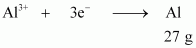
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al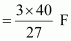
= 4.44 F
() According to the question,
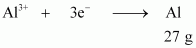
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al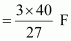
= 4.44 F
() According to the question,
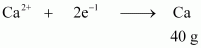
Electricity required to produce 40 g of calcium = 2 F
Therefore, electricity required to produce 20 g of calcium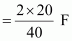
= 1 F
() According to the question,
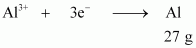
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al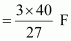
= 4.44 F
() According to the question,
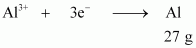
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al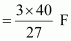
= 4.44 F
() According to the question,
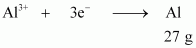
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al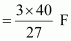
= 4.44 F
- #13-i20.0 g of Ca from molten CaCl2.
() 40.0 g of Al from molten Al2O3.
() 40.0 g of Al from molten Al2O3.Ans : According to the question,
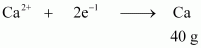
Electricity required to produce 40 g of calcium = 2 F
Therefore, electricity required to produce 20 g of calcium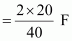
= 1 F
() According to the question,
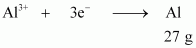
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al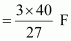
= 4.44 F
() According to the question,
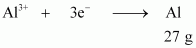
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al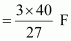
= 4.44 F
- #13-ii40.0 g of Al from molten Al2O3.Ans : According to the question,
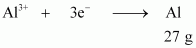
Electricity required to produce 27 g of Al = 3 F
Therefore, electricity required to produce 40 g of Al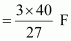
= 4.44 F