NEET-XII-Chemistry
03: Electrochemistry
- #11Conductivity of 0.00241 M acetic acid is 7.896 × 10-5 S cm-1. Calculate its molar conductivity and if
.gif) for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant?
for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant?
Ans : Given, κ = 7.896 × 10-5 S m-1
c = 0.00241 mol L-1
Then, molar conductivity,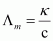
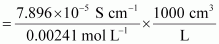
= 32.76S cm2 mol-1
Again,.gif) = 390.5 S cm2 mol-1
= 390.5 S cm2 mol-1
Now,
= 0.084
.gif) Dissociation constant,
Dissociation constant, 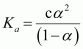
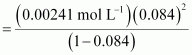
= 1.86 × 10-5 mol L-1