NEET-XII-Chemistry
03: Electrochemistry
- #12-i1 mol of Al3+ to Al.
() 1 mol of Cu2+ to Cu.
() 1 mol of to Mn2+.
to Mn2+.
() 1 mol of Cu2+ to Cu.
() 1 mol of to Mn2+.
to Mn2+.
() 1 mol of Cu2+ to Cu.
() 1 mol of to Mn2+.Ans :
to Mn2+.Ans :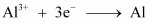
.gif) Required charge = 3 F
Required charge = 3 F
= 3 × 96487 C
= 289461 C
()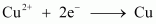
.gif) Required charge = 2 F
Required charge = 2 F
= 2 × 96487 C
= 192974 C
()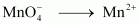
i.e.,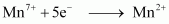
.gif) Required charge = 5 F
Required charge = 5 F
= 5 × 96487 C
= 482435 C
()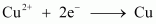
.gif) Required charge = 2 F
Required charge = 2 F
= 2 × 96487 C
= 192974 C
()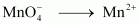
i.e.,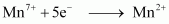
.gif) Required charge = 5 F
Required charge = 5 F
= 5 × 96487 C
= 482435 C
()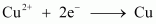
.gif) Required charge = 2 F
Required charge = 2 F
= 2 × 96487 C
= 192974 C
()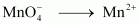
i.e.,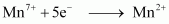
.gif) Required charge = 5 F
Required charge = 5 F
= 5 × 96487 C
= 482435 C
- #12-ii1 mol of Cu2+ to Cu.Ans :
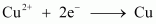
.gif) Required charge = 2 F
Required charge = 2 F
= 2 × 96487 C
= 192974 C
- #12-iii1 mol of
 to Mn2+.Ans :
to Mn2+.Ans :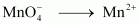
i.e.,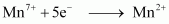
.gif) Required charge = 5 F
Required charge = 5 F
= 5 × 96487 C
= 482435 C