NEET-XII-Chemistry
03: Electrochemistry
- #10The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
Concentration/M 0.001 0.010 0.020 0.050 0.100
102 × κ/S m-1 1.237 11.85 23.15 55.53 106.74
Calculate.gif) for all concentrations and draw a plot between
for all concentrations and draw a plot between  and c½. Find the value of
and c½. Find the value of .
.
Ans : Given,
κ = 1.237 × 10-2 S m-1, c = 0.001 M
Then, κ = 1.237 × 10-4 S cm-1, c½ = 0.0316 M1/2
.gif)
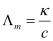
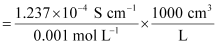
= 123.7 S cm2 mol-1
Given,
κ = 11.85 × 10-2 S m-1, c = 0.010M
Then, κ = 11.85 × 10-4 S cm-1, c½ = 0.1 M1/2
.gif)
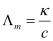
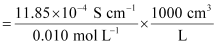
= 118.5 S cm2 mol-1
Given,
κ = 23.15 × 10-2 S m-1, c = 0.020 M
Then, κ = 23.15 × 10-4 S cm-1, c1/2 = 0.1414 M1/2
.gif)
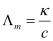
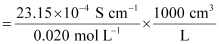
= 115.8 S cm2 mol-1
Given,
κ = 55.53 × 10-2 S m-1, c = 0.050 M
Then, κ = 55.53 × 10-4 S cm-1, c1/2 = 0.2236 M1/2
.gif)
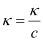
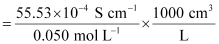
= 111.1 1 S cm2 mol-1
Given,
κ = 106.74 × 10-2 S m-1, c = 0.100 M
Then, κ = 106.74 × 10-4 S cm-1, c1/2 = 0.3162 M1/2
.gif)
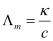
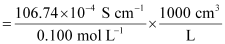
= 106.74 S cm2 mol-1
Now, we have the following data:
-
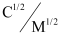
0.0316
0.1
0.1414
0.2236
0.3162
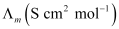
123.7
118.5
115.8
111.1
106.74
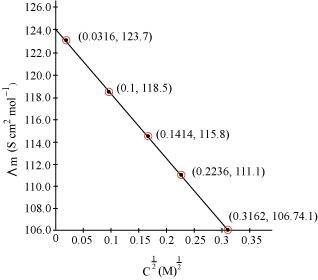
Since the line interrupts.gif) at 124.0 S cm2 mol-1,
at 124.0 S cm2 mol-1,  = 124.0 S cm2 mol-1.
= 124.0 S cm2 mol-1.
-