NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #15Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Ans : According to experimental results, the dipole moment of carbon dioxide is zero. This is possible only if the molecule is linear so that the dipole moments of C-O bonds are equal and opposite to nullify each other.
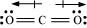
Resultant μ = 0 D
H2O, on the other hand, has a dipole moment value of 1.84 D (though it is a triatomic molecule as CO2). The value of the dipole moment suggests that the structure of H2O molecule is bent where the dipole moment of O-H bonds are unequal.
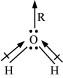
- Qstn #16Write the significance/applications of dipole moment.
Ans : In heteronuclear molecules, polarization arises due to a difference in the electronegativities of the constituents of atoms. As a result, one end of the molecule acquires a positive charge while the other end becomes negative. Hence, a molecule is said to possess a dipole.
The product of the magnitude of the charge and the distance between the centres of positive-negative charges is called the dipole moment (μ) of the molecule. It is a vector quantity and is represented by an arrow with its tail at the positive centre and head pointing towards a negative centre.
Dipole moment (μ) = charge (Q) × distance of separation (r)
The SI unit of a dipole moment is ‘esu’.
1 esu = 3.335 × 10-30Cm
Dipole moment is the measure of the polarity of a bond. It is used to differentiate between polar and non-polar bonds since all non-polar molecules (e.g. H2, O2) have zero dipole moments. It is also helpful in calculating the percentage ionic character of a molecule.
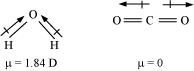
- Qstn #17Define electronegativity. How does it differ from electron gain enthalpy?
Ans : Electronegativity is the ability of an atom in a chemical compound to attract a bond pair of electrons towards itself.
Electronegativity of any given element is not constant. It varies according to the element to which it is bound. It is not a measurable quantity. It is only a relative number.
On the other hand, electron gain enthalpy is the enthalpy change that takes place when an electron is added to a neutral gaseous atom to form an anion. It can be negative or positive depending upon whether the electron is added or removed. An element has a constant value of the electron gain enthalpy that can be measured experimentally.
- Qstn #18Explain with the help of suitable example polar covalent bond.
Ans : When two dissimilar atoms having different electronegativities combine to form a covalent bond, the bond pair of electrons is not shared equally. The bond pair shifts towards the nucleus of the atom having greater electronegativity. As a result, electron distribution gets distorted and the electron cloud is displaced towards the electronegative atom.
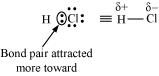
As a result, the electronegative atom becomes slightly negatively charged while the other atom becomes slightly positively charged. Thus, opposite poles are developed in the molecule and this type of a bond is called a polar covalent bond.
HCl, for example, contains a polar covalent bond. Chlorine atom is more electronegative than hydrogen atom. Hence, the bond pair lies towards chlorine and therefore, it acquires a partial negative charge.
- Qstn #19Arrange the bonds in order of increasing ionic character in the molecules: LiF, K2O, N2, SO2 and ClF3.
Ans : The ionic character in a molecule is dependent upon the electronegativity difference between the constituting atoms. The greater the difference, the greater will be the ionic character of the molecule.
On this basis, the order of increasing ionic character in the given molecules is
N2 < SO2 < ClF3 < K2O < LiF.
- Qstn #20The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
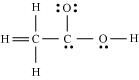
Ans : The correct Lewis structure for acetic acid is as follows:
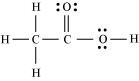
- Qstn #21Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
Ans : Electronic configuration of carbon atom:
6C: 1s2 2s2 2p2
In the excited state, the orbital picture of carbon can be represented as:
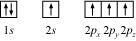
Hence, carbon atom undergoes sp3 hybridization in CH4 molecule and takes a tetrahedral shape.
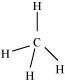
For a square planar shape, the hybridization of the central atom has to be dsp2. However, an atom of carbon does not have d-orbitalsto undergo dsp2 hybridization. Hence, the structure of CH4 cannot be square planar.
Moreover, with a bond angle of 90° in square planar, the stability of CH4 will be very less because of the repulsion existing between the bond pairs. Hence, VSEPR theory also supports a tetrahedral structure for CH4.
- Qstn #22Explain why BeH2 molecule has a zero dipole moment although the Be-H bonds are polar.
Ans : The Lewis structure for BeH2 is as follows:
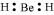
There is no lone pair at the central atom (Be) and there are two bond pairs. Hence, BeH2 is of the type AB2. It has a linear structure.
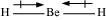
Dipole moments of each H-Be bond are equal and are in opposite directions. Therefore, they nullify each other. Hence, BeH2 molecule has zero dipole moment.
- Qstn #23Which out of NH3 and NF3 has higher dipole moment and why?
Ans : In both molecules i.e., NH3 and NF3, the central atom (N) has a lone pair electron and there are three bond pairs. Hence, both molecules have a pyramidal shape. Since fluorine is more electronegative than hydrogen, it is expected that the net dipole moment of NF3 is greater than NH3. However, the net dipole moment of NH3 (1.46 D) is greater than that of NF3 (0.24 D).
This can be explained on the basis of the directions of the dipole moments of each individual bond in NF3 and NH3. These directions can be shown as:
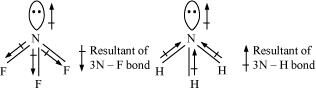
Thus, the resultant moment of the N-H bonds add up to the bond moment of the lone pair (the two being in the same direction), whereas that of the three N - F bonds partly cancels the moment of the lone pair.
Hence, the net dipole moment of NF3 is less than that of NH3.
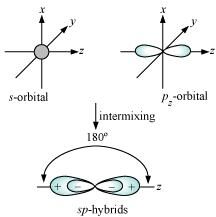
- Qstn #24What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
Ans : Hybridization is defined as an intermixing of a set of atomic orbitals of slightly different energies, thereby forming a new set of orbitals having equivalent energies and shapes.
For example, one 2s-orbital hybridizes with two 2p-orbitals of carbon to form three new sp2 hybrid orbitals.
These hybrid orbitals have minimum repulsion between their electron pairs and thus, are more stable. Hybridization helps indicate the geometry of the molecule.
Shape of sp hybrid orbitals: sp hybrid orbitals have a linear shape. They are formed by the intermixing of s and p orbitals as:
Shape of sp2 hybrid orbitals:
sp2 hybrid orbitals are formed as a result of the intermixing of one s-orbital and two 2p-orbitals. The hybrid orbitals are oriented in a trigonal planar arrangement as:
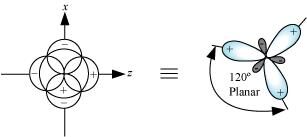
Shape of sp3 hybrid orbitals:
Four sp3 hybrid orbitals are formed by intermixing one s-orbital with three p-orbitals. The four sp3 hybrid orbitals are arranged in the form of a tetrahedron as:
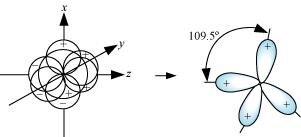
- Qstn #25Describe the change in hybridisation (if any) of the Al atom in the following reaction.

Ans : The valence orbital picture of aluminium in the ground state can be represented as:
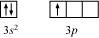
The orbital picture of aluminium in the excited state can be represented as:

Hence, it undergoes sp2 hybridization to give a trigonal planar arrangement (in AlCl3).
To form AlCl4-, the empty 3pz orbital also gets involved and the hybridization changes from sp2 to sp3. As a result, the shape gets changed to tetrahedral.
- Qstn #26Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
BF3 + NH3 → F3B.NH3
Ans : Boron atom in BF3 is sp2 hybridized. The orbital picture of boron in the excited state can be shown as:
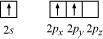
Nitrogen atom in NH3 is sp3 hybridized. The orbital picture of nitrogen can be represented as:

After the reaction has occurred, an adduct F3B⋅NH3 is formed as hybridization of ‘B’ changes to sp3. However, the hybridization of ‘N’ remains intact.
- Qstn #27Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Ans : C2H4 :
The electronic configuration of C-atom in the excited state is:
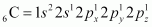
In the formation of an ethane molecule (C2H4), one sp2 hybrid orbital of carbon overlaps a sp2 hybridized orbital of another carbon atom, thereby forming a C-C sigma bond.
The remaining two sp2 orbitals of each carbon atom form a sp2-s sigma bond with two hydrogen atoms. The unhybridized orbital of one carbon atom undergoes sidewise overlap with the orbital of a similar kind present on another carbon atom to form a weak ``\pi``-bond.
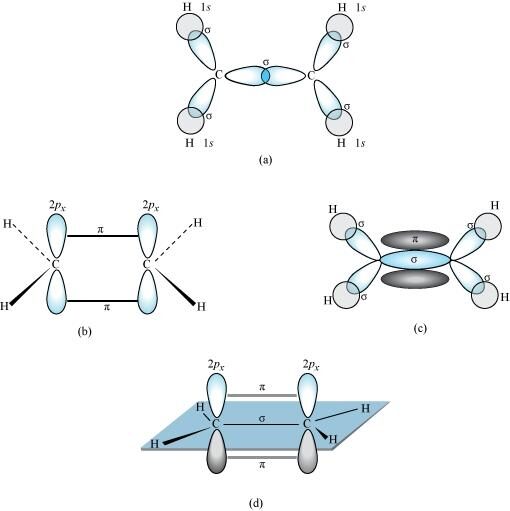
C2H2 :
In the formation of C2H2 molecule, each C-atom is sp hybridized with two 2p-orbitals in an unhybridized state.
One sp orbital of each carbon atom overlaps with the other along the internuclear axis forming a C-C sigma bond. The second sp orbital of each C-atom overlaps a half-filled 1s-orbital to form a σ bond.
The two unhybridized 2p-orbitals of the first carbon undergo sidewise overlap with the 2p orbital of another carbon atom, thereby forming two pi (``\pi``) bonds between carbon atoms. Hence, the triple bond between two carbon atoms is made up of one sigma and two ``\pi``-bonds.
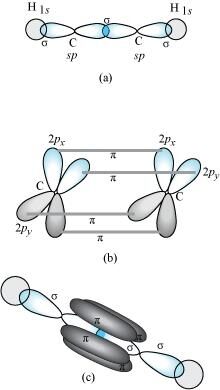
- Qstn #28What is the total number of sigma and pi bonds in the following molecules?
(a) C2H2 (b) C2H4Ans : A single bond is a result of the axial overlap of bonding orbitals. Hence, it contributes a sigma bond. A multiple bond (double or triple bond) is always formed as a result of the sidewise overlap of orbitals. A pi-bond is always present in it. A triple bond is a combination of two pi-bonds and one sigma bond.
Structure of C2H2 can be represented as:
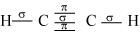
Hence, there are three sigma and two pi-bonds in C2H2.
The structure of C2H4 can be represented as:
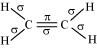
Hence, there are five sigma bonds and one pi-bond in C2H4.
- Qstn #29Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why? (a) 1s and 1s (b) 1s and 2px (c) 2py and 2py (d) 1s and 2s.
Ans : 2py and 2py orbitals will not a form a sigma bond. Taking x-axis as the internuclear axis, 2py and 2py orbitals will undergo lateral overlapping, thereby forming a pi (``\pi``) bond.