NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Qstn #30Which hybrid orbitals are used by carbon atoms in the following molecules?
CH3-CH3; (b) CH3-CH=CH2; (c) CH3-CH2-OH; (d) CH3-CHO (e) CH3COOH
Ans : (a)
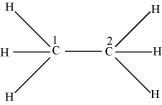
Both C1 and C2 are sp3 hybridized.
(b)
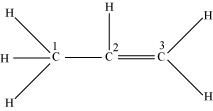
C1 is sp3 hybridized, while C2 and C3 are sp2 hybridized.
(c)
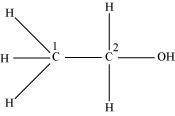
Both C1 and C2 are sp3 hybridized.
(d)
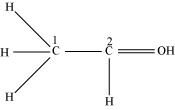
C1 is sp3 hybridized and C2 is sp2 hybridized.
(e)
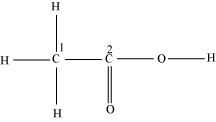
C1 is sp3 hybridized and C2 is sp2 hybridized.
- Qstn #31What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
Ans : When two atoms combine by sharing their one or more valence electrons, a covalent bond is formed between them.
The shared pairs of electrons present between the bonded atoms are called bond pairs. All valence electrons may not participate in bonding. The electron pairs that do not participate in bonding are called lone pairs of electrons.
For example, in C2H6 (ethane), there are seven bond pairs but no lone pair present.
In H2O, there are two bond pairs and two lone pairs on the central atom (oxygen).
- Qstn #32Distinguish between a sigma and a pi bond.
Ans : The following are the differences between sigma and pi-bonds:
Sigma (σ) Bond
Pi (``\pi``) Bond
(a) It is formed by the end to end overlap of orbitals. It is formed by the lateral overlap of orbitals. (b) The orbitals involved in the overlapping are s-s, s-p, or p-p. These bonds are formed by the overlap of p-p orbitals only. (c) It is a strong bond. It is weak bond. (d) The electron cloud is symmetrical about the line joining the two nuclei. The electron cloud is not symmetrical. (e) It consists of one electron cloud, which is symmetrical about the internuclear axis. There are two electron clouds lying above and below the plane of the atomic nuclei. (f) Free rotation about σ bonds is possible. Rotation is restricted in case of pi-bonds.
- Qstn #33Explain the formation of H2 molecule on the basis of valence bond theory.
Ans : Let us assume that two hydrogen atoms (A and B) with nuclei (NA and NB) and electrons (eA and eB) are taken to undergo a reaction to form a hydrogen molecule.
When A and B are at a large distance, there is no interaction between them. As they begin to approach each other, the attractive and repulsive forces start operating.
Attractive force arises between:
(a) Nucleus of one atom and its own electron i.e., NA - eA and NB - eB.
(b) Nucleus of one atom and electron of another atom i.e., NA - eB and NB - eA.
Repulsive force arises between:
(a) Electrons of two atoms i.e., eA - eB.
(b) Nuclei of two atoms i.e., NA - NB.
The force of attraction brings the two atoms together, whereas the force of repulsion tends to push them apart.
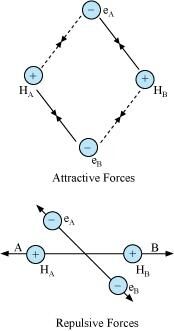
The magnitude of the attractive forces is more than that of the repulsive forces. Hence, the two atoms approach each other. As a result, the potential energy decreases. Finally, a state is reached when the attractive forces balance the repulsive forces and the system acquires minimum energy. This leads to the formation of a dihydrogen molecule.
- Qstn #34Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Ans : The given conditions should be satisfied by atomic orbitals to form molecular orbitals:
(a) The combining atomic orbitals must have the same or nearly the same energy. This means that in a homonuclear molecule, the 1s-atomic orbital of an atom can combine with the 1s-atomic orbital of another atom, and not with the 2s-orbital.
(b) The combining atomic orbitals must have proper orientations to ensure that the overlap is maximum.
(c) The extent of overlapping should be large.
- Qstn #35Use molecular orbital theory to explain why the Be2 molecule does not exist.
Ans : The electronic configuration of Beryllium is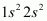 .
.
The molecular orbital electronic configuration for Be2 molecule can be written as:
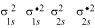
Hence, the bond order for Be2 is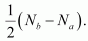
Where,
Nb = Number of electrons in bonding orbitals
Na = Number of electrons in anti-bonding orbitals
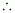 Bond order of Be2
Bond order of Be2 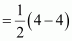 = 0
= 0
A negative or zero bond order means that the molecule is unstable. Hence, Be2 molecule does not exist.
- Qstn #36Compare the relative stability of the following species and indicate their magnetic properties;
O2,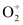 ,
,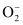 (superoxide),
(superoxide), 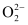 (peroxide)
(peroxide)
Ans : There are 16 electrons in a molecule of dioxygen, 8 from each oxygen atom. The electronic configuration of oxygen molecule can be written as:
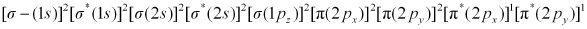
Since the 1s orbital of each oxygen atom is not involved in boding, the number of bonding electrons = 8 = Nb and the number of anti-bonding orbitals = 4 = Na.
Bond order
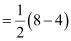
= 2
Similarly, the electronic configuration of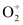 can be written as:
can be written as:
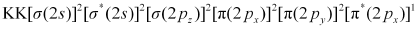
Nb = 8
Na = 3
Bond order of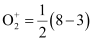
= 2.5
Electronic configuration of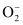 ion will be:
ion will be:
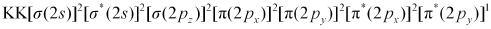
Nb = 8
Na = 5
Bond order of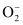 =
= 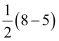
= 1.5
Electronic configuration of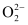 ion will be:
ion will be:
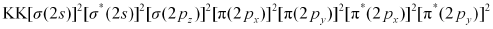
Nb = 8
Na = 6
Bond order of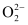 =
= 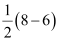
= 1
Bond dissociation energy is directly proportional to bond order. Thus, the higher the bond order, the greater will be the stability. On this basis, the order of stability is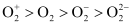 .
.
- Qstn #37Write the significance of a plus and a minus sign shown in representing the orbitals.
Ans : Molecular orbitals are represented by wave functions. A plus sign in an orbital indicates a positive wave function while a minus sign in an orbital represents a negative wave function.
- Qstn #38Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
Ans : The ground state and excited state outer electronic configurations of phosphorus (Z = 15) are:
.png)
Phosphorus atom is sp3d hybridized in the excited state. These orbitals are filled by the electron pairs donated by five Cl atoms as:
PCl5
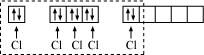
The five sp3d hybrid orbitals are directed towards the five corners of the trigonal bipyramidals. Hence, the geometry of PCl5 can be represented as:
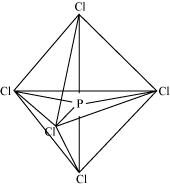
There are five P-Cl sigma bonds in PCl5. Three P-Cl bonds lie in one plane and make an angle of 120° with each other. These bonds are called equatorial bonds.
The remaining two P-Cl bonds lie above and below the equatorial plane and make an angle of 90° with the plane. These bonds are called axial bonds.
As the axial bond pairs suffer more repulsion from the equatorial bond pairs, axial bonds are slightly longer than equatorial bonds.
- Qstn #39Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Ans : A hydrogen bond is defined as an attractive force acting between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule (may be of the same kind).
Due to a difference between electronegativities, the bond pair between hydrogen and the electronegative atom gets drifted far away from the hydrogen atom. As a result, a hydrogen atom becomes electropositive with respect to the other atom and acquires a positive charge.
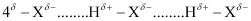
The magnitude of H-bonding is maximum in the solid state and minimum in the gaseous state.
There are two types of H-bonds:
(i) Intermolecular H-bond e.g., HF, H2O etc.
(ii) Intramolecular H-bond e.g., o-nitrophenol
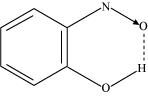
Hydrogen bonds are stronger than Van der Walls forces since hydrogen bonds are regarded as an extreme form of dipole-dipole interaction.
- Qstn #40What is meant by the term bond order? Calculate the bond order of: N2, O2,
.gif) and
and.gif) .
.
Ans : Bond order is defined as one half of the difference between the number of electrons present in the bonding and anti-bonding orbitals of a molecule.
If Na is equal to the number of electrons in an anti-bonding orbital, then Nb is equal to the number of electrons in a bonding orbital.
Bond order =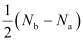
If Nb > Na, then the molecule is said be stable. However, if Nb ≤ Na, then the molecule is considered to be unstable.
Bond order of N2 can be calculated from its electronic configuration as:
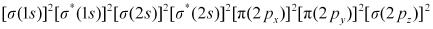
Number of bonding electrons, Nb = 10
Number of anti-bonding electrons, Na = 4
Bond order of nitrogen molecule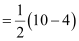
= 3
There are 16 electrons in a dioxygen molecule, 8 from each oxygen atom. The electronic configuration of oxygen molecule can be written as:
.gif)
Since the 1s orbital of each oxygen atom is not involved in boding, the number of bonding electrons = 8 = Nb and the number of anti-bonding electrons = 4 = Na.
Bond order.gif)
.gif)
= 2
Hence, the bond order of oxygen molecule is 2.
Similarly, the electronic configuration of.gif) can be written as:
can be written as:
.gif)
Nb = 8
Na = 3
Bond order of.gif)
= 2.5
Thus, the bond order of.gif) is 2.5.
is 2.5.
The electronic configuration of.gif) ion will be:
ion will be:
.gif)
Nb = 8
Na = 5
Bond order of.gif) =
= .gif)
= 1.5
Thus, the bond order of.gif) ion is 1.5.
ion is 1.5.