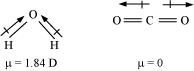NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #16Write the significance/applications of dipole moment.
Ans : In heteronuclear molecules, polarization arises due to a difference in the electronegativities of the constituents of atoms. As a result, one end of the molecule acquires a positive charge while the other end becomes negative. Hence, a molecule is said to possess a dipole.
The product of the magnitude of the charge and the distance between the centres of positive-negative charges is called the dipole moment (μ) of the molecule. It is a vector quantity and is represented by an arrow with its tail at the positive centre and head pointing towards a negative centre.
Dipole moment (μ) = charge (Q) × distance of separation (r)
The SI unit of a dipole moment is ‘esu’.
1 esu = 3.335 × 10-30Cm
Dipole moment is the measure of the polarity of a bond. It is used to differentiate between polar and non-polar bonds since all non-polar molecules (e.g. H2, O2) have zero dipole moments. It is also helpful in calculating the percentage ionic character of a molecule.