NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #27Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Ans : C2H4 :
The electronic configuration of C-atom in the excited state is:
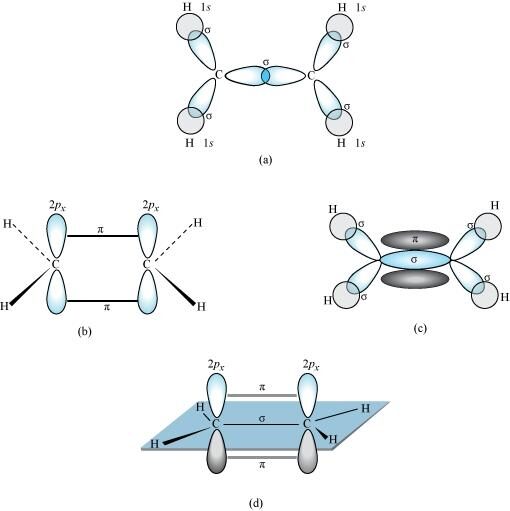
In the formation of an ethane molecule (C2H4), one sp2 hybrid orbital of carbon overlaps a sp2 hybridized orbital of another carbon atom, thereby forming a C-C sigma bond.
The remaining two sp2 orbitals of each carbon atom form a sp2-s sigma bond with two hydrogen atoms. The unhybridized orbital of one carbon atom undergoes sidewise overlap with the orbital of a similar kind present on another carbon atom to form a weak ``\pi``-bond.
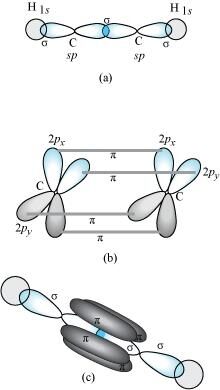
C2H2 :
In the formation of C2H2 molecule, each C-atom is sp hybridized with two 2p-orbitals in an unhybridized state.
One sp orbital of each carbon atom overlaps with the other along the internuclear axis forming a C-C sigma bond. The second sp orbital of each C-atom overlaps a half-filled 1s-orbital to form a σ bond.
The two unhybridized 2p-orbitals of the first carbon undergo sidewise overlap with the 2p orbital of another carbon atom, thereby forming two pi (``\pi``) bonds between carbon atoms. Hence, the triple bond between two carbon atoms is made up of one sigma and two ``\pi``-bonds.