NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #15Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Ans : According to experimental results, the dipole moment of carbon dioxide is zero. This is possible only if the molecule is linear so that the dipole moments of C-O bonds are equal and opposite to nullify each other.
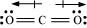
Resultant μ = 0 D
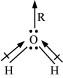
H2O, on the other hand, has a dipole moment value of 1.84 D (though it is a triatomic molecule as CO2). The value of the dipole moment suggests that the structure of H2O molecule is bent where the dipole moment of O-H bonds are unequal.