NEET-XII-Chemistry
08: The d-and f-Block Elements
- Qstn #10What are the different oxidation states exhibited by the lanthanoids?
Ans : In the lanthanide series, +3 oxidation state is most common i.e., Ln(III) compounds are predominant. However, +2 and +4 oxidation states can also be found in the solution or in solid compounds.
- Qstn #11-iTransition metals and many of their compounds show paramagnetic
behaviour.
() The enthalpies of atomisation of the transition metals are high.
() The transition metals generally form coloured compounds.
() Transition metals and their many compounds act as good catalyst.
() The enthalpies of atomisation of the transition metals are high.
() The transition metals generally form coloured compounds.
() Transition metals and their many compounds act as good catalyst.Ans : Transition metals show paramagnetic behaviour. Paramagnetism arises due to the presence of unpaired electrons with each electron having a magnetic moment associated with its spin angular momentum and orbital angular momentum. However, in the first transition series, the orbital angular momentum is quenched. Therefore, the resulting paramagnetism is only because of the unpaired electron.
() Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high.
() Most of the complexes of transition metals are coloured. This is because of the absorption of radiation from visible light region to promote an electron from one of the d-orbitals to another. In the presence of ligands, the d-orbitals split up into two sets of orbitals having different energies. Therefore, the transition of electrons can take place from one set toanother. The energy required for these transitions is quite small and falls in the visible region of radiation. The ions of transition metals absorb the radiation of a particular wavelength and the rest is reflected, imparting colour to the solution.
() The catalytic activity of the transition elements can be explained by two basic facts.
(a) Owing to their ability to show variable oxidation states and form complexes, transition metals form unstable intermediate compounds. Thus, they provide a new path with lower activation energy, Ea, for the reaction.
(b) Transition metals also provide a suitable surface for the reactions to occur.
() Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high.
() Most of the complexes of transition metals are coloured. This is because of the absorption of radiation from visible light region to promote an electron from one of the d-orbitals to another. In the presence of ligands, the d-orbitals split up into two sets of orbitals having different energies. Therefore, the transition of electrons can take place from one set toanother. The energy required for these transitions is quite small and falls in the visible region of radiation. The ions of transition metals absorb the radiation of a particular wavelength and the rest is reflected, imparting colour to the solution.
() The catalytic activity of the transition elements can be explained by two basic facts.
(a) Owing to their ability to show variable oxidation states and form complexes, transition metals form unstable intermediate compounds. Thus, they provide a new path with lower activation energy, Ea, for the reaction.
(b) Transition metals also provide a suitable surface for the reactions to occur.
- Qstn #11-iiThe enthalpies of atomisation of the transition metals are high.Ans : Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high.
- Qstn #11-iiiThe transition metals generally form coloured compounds.Ans : Most of the complexes of transition metals are coloured. This is because of the absorption of radiation from visible light region to promote an electron from one of the d-orbitals to another. In the presence of ligands, the d-orbitals split up into two sets of orbitals having different energies. Therefore, the transition of electrons can take place from one set toanother. The energy required for these transitions is quite small and falls in the visible region of radiation. The ions of transition metals absorb the radiation of a particular wavelength and the rest is reflected, imparting colour to the solution.
- Qstn #11-ivTransition metals and their many compounds act as good catalyst.Ans : The catalytic activity of the transition elements can be explained by two basic facts.
(a) Owing to their ability to show variable oxidation states and form complexes, transition metals form unstable intermediate compounds. Thus, they provide a new path with lower activation energy, Ea, for the reaction.
(b) Transition metals also provide a suitable surface for the reactions to occur.
- Qstn #12What are interstitial compounds? Why are such compounds well known for
transition metals?
Ans : Transition metals are large in size and contain lots of interstitial sites. Transition elements can trap atoms of other elements (that have small atomic size), such as H, C, N, in the interstitial sites of their crystal lattices. The resulting compounds are called interstitial compounds.
- Qstn #13How is the variability in oxidation states of transition metals different from
that of the non-transition metals? Illustrate with examples.
Ans : In transition elements, the oxidation state can vary from +1 to the highest oxidation state by removing all its valence electrons. Also, in transition elements, the oxidation states differ by 1 (Fe2+ and Fe3+; Cu+ and Cu2+). In non-transition elements, the oxidation states differ by 2, for example, +2 and +4 or +3 and +5, etc.
- Qstn #14Describe the preparation of potassium dichromate from iron chromite ore.
What is the effect of increasing pH on a solution of potassium dichromate?
Ans : Potassium dichromate is prepared from chromite ore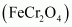 in the following steps.
in the following steps.
Step (1): Preparation of sodium chromate
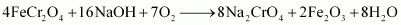
Step (2): Conversion of sodium chromate into sodium dichromate

Step(3): Conversion of sodium dichromate to potassium dichromate

Potassium dichromate being less soluble than sodium chloride is obtained in the form of orange coloured crystals and can be removed by filtration.
The dichromate ion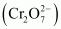 exists in equilibrium with chromate
exists in equilibrium with chromate 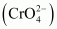 ion at pH 4. However, by changing the pH, they can be interconverted.
ion at pH 4. However, by changing the pH, they can be interconverted.
2CrO42-→Acid2HCrO4-→Acid2Cr2O72-Cr2O72-→Base2HCrO42-→Base2CrO42-
- Qstn #15Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
Ans : acts as a very strong oxidising agent in the acidic medium.
acts as a very strong oxidising agent in the acidic medium.
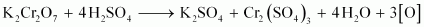
 takes up electrons to get reduced and acts as an oxidising agent. The reaction of K2Cr2O7 with other iodide, iron (II) solution, and H2S are given below.
takes up electrons to get reduced and acts as an oxidising agent. The reaction of K2Cr2O7 with other iodide, iron (II) solution, and H2S are given below.
- Qstn #15-iiiron(II) solution andAns :
 oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
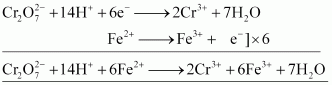
- Qstn #16Describe the preparation of potassium permanganate. How does the acidified permanganate solution react withAns : Potassium permanganate can be prepared from pyrolusite (MnO2). The ore is fused with KOH in the presence of either atmospheric oxygen or an oxidising agent, such as KNO3 or KClO4, to give K2MnO4.
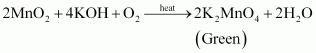
The green mass can be extracted with water and then oxidized either electrolytically or by passing chlorine/ozone into the solution.
Electrolytic oxidation
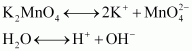
At anode, manganate ions are oxidized to permanganate ions.
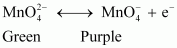
Oxidation by chlorine
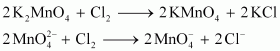
Oxidation by ozone
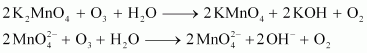
- Qstn #16-iiron(II) ionsAns : Acidified KMnO4 solution oxidizes Fe (II) ions to Fe (III) ions i.e., ferrous ions to ferric ions.